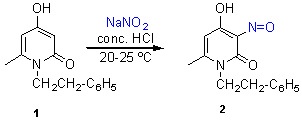
4-Hydroxy-6-methyl-3-nitroso-1-phenethyl-2(1H)-pyridinone
Edmont V. Stoyanov* and Ivo C. Ivanov
Faculty of Pharmacy, Medical University of Sofia, Dunav 2, BG-1000 Sofia, Bulgaria, Tel. (+359 2) 988 3142, Fax (+359 2) 987 9874, E-mail: [email protected]
Received: 10 January 2000 / Accepted: 14 January 2000 / Published: 24 January 2000
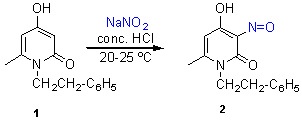
The starting 4-hydroxy-6-methyl-1-phenethyl-2(1H)-pyridinone (1) was prepared according to the known procedure [1,2]. To a suspension of the pyridinone 1 (2.29 g, 10 mmol) in concentrated hydrochloric acid (15 ml), was added dropwise a solution of sodium nitrite (620 mg, 9 mmol) in water (8 ml) with stirring at 20-25°C. The reaction mixture was stirred for further 10 min. The crystals that separated were filtered off, washed twice with cold water and dried (2 h at 70-80°C) to afford the title compound 2. Yield after recrystallization from methanol: 1.71 g (71 %). Brown needles, m.p. 148 ºC (dec.) (methanol).
1H NMR (100 MHz, CDCl3): 2.10 (s, 3H, 6-CH3), 2.99 (t, J = 7.0 Hz, 2H, 1-CH2CH2Ph), 4.11 (t, J = 7.7 Hz, 2H, 1-CH2CH2Ph), 5.63 (s, 1H, H-5), 7.26 (m, 5H arom., C6H5).
FT IR (nujol): 1700, 1634, 1578, 1540, 1364, 1308, 1256, 1183, 1152, 1111, 1082, 974, 833, 750, 702.
EI MS (70 eV; m/z (%)): 258 (M+., 12), 244 (15), 242 (8), 227 (3), 214 (8), 201 (1), 197 (2), 186 (7), 154 (18), 140 (92), 123 (16), 110 (36), 105 (56), 104 (100), 96 (63), 91 (30), 77 (34), 65 (19), 55 (34), 44 (90).
Anal. calcd. for C14H14N2O3 (258.27): C 65.10, H 5.50, N 10.80; Found C 64.84, H 5.46, N 10.83.
References
| 1. | Ivanov, I. C.; Stoyanov, E. V.; Alexandrova, S. V. Farmatsiya (Sofia) 1997, 44 (2), 3-6; C.A. 1998, 128, 243925w. |
| 2. | Castillo, S.; Ouadahi, H.; Herault, V. Bull. Soc. Chim. Fr. 1982, II-257-261. |
Sample Availability: Available from the authors and from MDPI.
©2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/