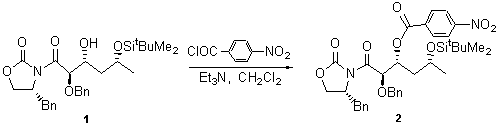
3-[5-(tert-Butyldimethylsilyloxy)-3-p-nitrobenzoyl-1-oxo- 2-(phenylmethoxy)hexyl]-4-(phenylmethyl)-2-oxazolidinone
Margaret A. Brimble* and Josephine S. O. Park
School of Chemistry, University of Sydney, Eastern Ave, Camperdown, NSW 2006, Australia. Fax: (+61 3) 9351 3329; E-mail: [email protected]
Received: 20 January 2000 / Accepted: 2 February 2000 / Published: 23 February 2000
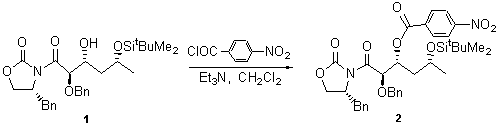
A mixture of alcohol 1 (121 mg, 0.23 mmol) [1], triethylamine (48 l, 0.34 mmol) and p-nitrobenzoyl chloride (51 mg, 0.28 mmol) in dichloromethane (2 ml) was stirred for 2 h. at 30ºC. The reaction mixture was poured into saturated aqueous sodium hydrogen carbonate (5 ml), extracted into ethyl acetate (2 x 10 ml), washed with water (2 x 5 ml) and dried over sodium sulfate. Removal of the solvent under reduced pressure and purification of the residue by flash chromatography, using light petroleum-ethyl acetate (8:2) as eluent afforded the title compound 2 (129 mg, 83%) as a colourless oil.
[a]D -41.34 (c 1.024, CHCl3).
IR (cm-1, neat): 1783s, 1729s, 1714s, 1386m, 1103m.
1H NMR (400 MHz, CDCl3): 0.01, 0.02 (6H, s, SiMe2), 0.85 (9H, s, But), 1.21 (3H, d, J6',5' 6.0 Hz, H6'), 2.10-2.14 (2H, m, H4'), 2.62 (1H, dd, Jgem 13.5 and J 9.5 Hz, CHCHAPh), 3.15 (1H, dd, Jgem 13.5 and J 3.3 Hz, CHCHBPh), 3.76 (1H, dd, Jgem 8.8 and J5A,4 8.8 Hz, H5A), 3.95-4.00 (1H, m, H5'), 4.06 (1H, dd, Jgem 8.8 and J5B,4 2.5 Hz, H5B), 4.47-4.51 (1H, m, H4), 4.70 (2H, s, OCH2Ph), 5.55-5.60 (2H, m, H2', H3'), 7.15-7.42 (10H, m, Ph), 8.18 (2H, d, J 8.8 Hz, PhNO2), 8.27 (2H, d, J 8.8 Hz, PhNO2).
13C NMR (100 MHz, CDCl3): -4.1, -3.9 (CH3, SiMe2), 18.8 (quat., CMe3), 24.0 (CH3, C6'), 25.5 (CH3, CMe3), 38.3 (CH2, CHCH2Ph), 41.5 (CH2, C4'), 56.2 (CH, C4), 66.3 (CH2, C5), 67.1 (CH, C5'), 73.5 (CH, C3'), 74.2 (CH2, OCH2Ph), 78.5 (CH, C2'), 124.2, 124.3, 128.1, 128.9, 129.2, 129.6, 130.1, 131.3, 131.5 [CH, 3 x Ph (last 5 peaks coincidental)], 135.5 (quat., CHCH2Ph), 135.7 (quat., OC=OC), 137.6 (quat., OCH2Ph), 151.4 (quat., CNO2), 153.6 (quat., C2) 164.0 (quat., OC=O), 170.7 (quat., C1').
CI-MS: (FAB, NBA matrix) 677 (MH+, 2%), 619 (4), 569 (MH+-HOCH2Ph, 1), 545 (MH+-HOSiMe2But, 4), 224 (5), 178 (C10H12NO2, 6), 159 (C8H19OSi, 9), 150 (15), 136 (14), 91 (CH2Ph, 100), 73 (27).
Anal. calc. for C36H44N2O9Si MH+ (CI, NH3), 677.2901; found MH+, 677.2894.
Reference
| 1. | Brimble, M. A.; Park, J. S. O. J. Chem. Soc. Perkin Trans. I 2000, 697-709. |
Sample availability: available from the authors.
©2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/