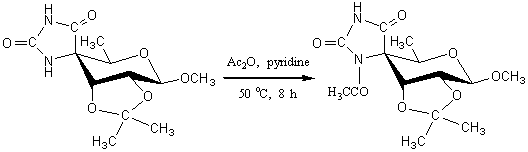
(4R)-1'-Acetyl-2,3-O-isopropylidene-methylspiro [4,6-dideoxy-ß-D-ribo-hexopyranosid-4,5'-imidazolidin]-2',4'-dione
Bohumil Steiner, Jan Gajdos and Miroslav Koos*
Institute of Chemistry, Slovak Academy of Sciences, Dubravska 9, SK-84238 Bratislava, Slovakia Tel. +421-7-59410254, Fax +421-7-59410222 (E-mail: [email protected])
Received: 17 January 2000 / Accepted: 7 February 2000 / Published: 23 February 2000
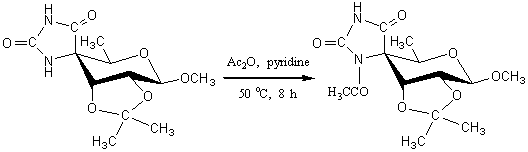
Acetylation of hydantoins (imidazolidin-2,4-diones) and their 5-substituted derivatives normally occurs more readily in the 1-position [1]. A mixture of (4R)-2,3-O-isopropylidene-methylspiro[4,6-dideoxy-ß-D-ribo -hexopyranosid-4,5'-imidazolidin]-2',4'-dione [2] (0.86 g, 3 mmol), acetic anhydride (2.5 ml) and dry pyridine (5 ml) was heated at 50 °C for 8 h followed by concentration and co-evaporation with toluene under diminished pressure to give the crude product. This was purified on a column of silica gel using CHCl3/MeOH 4:1 as an eluent. The fractions with Rf 0.35 were collected and evaporated to afford the title compound (0.87 g, 89%) as a colourless oil which solidified on standing.
M.p. 98-99 °C.
[a]D 8° (c = 10 mg.cm3, methanol).
TLC (CHCl3/MeOH 4:1, silica gel) Rf 0.35.
1H-NMR (CDCl3): 5.26 (q, J=6.5 Hz, 1H, H-5); 4.54 (d, J=7.3 Hz, 1H, H-1); 4.37 (d, J=5.7 Hz, 1H, H-3); 4.27 (dd, J=7.3 and 5.7 Hz, 1H, H-2); 3.56 (s, 3H, OMe); 2.58 (s, 3H, COMe); 1.53 and 1.33 (2s, 2 x 3H, CMe2); 1.27 (d, J=6.5 Hz, 3H, H-6).
13C-NMR (CDCl3): 169.9, 169.5 and 153.5 (3 x C=O), 111.0 (CMe2), 103.5 (C-1), 76.3 (C-2), 76.2 (C-3), 70.2 (C-4), 68.0 (C-5), 57.2 (OMe), 27.6 and 25.8 (CMe2), 27.0 (COMe), 14.8 (C-6).
CI-MS (70 eV, pyridine): m/z 408 (100%, [M + C5H5NH]+).
Anal. calc. for C14H20N2O7 (328.32): C 51.22, H 6.14, N 8.53; found: C 51.11, H 6.18, N 8.59.
Acknowledgments: The authors would like to thank Scientific Grant Agency (VEGA, project No. 2/4144/99 and 2/7144/20) for financial support.
References
| 1. | Theobald, R. S. In Rodd's Chemistry of Carbon Compounds, Second Edition, Coffey, S. and Ansell, M. F., Eds., Elsevier: Amsterdam, 1986; Vol. IV, Part C, p 191. |
| 2. | Koos, M.; Steiner, B.; Langer, V.; Gyepesova, D.; Durik, M. Carbohydr. Res., in press. |
Sample availability: Available from authors.
©2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/