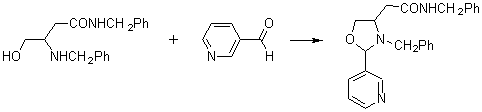
3-Benzyl-4-(N-benzylcarbamoylmethyl)-2-(3-pyridyl)-1,3-oxazolidine
Marina A. Tlekhusezh, Roman V. Makuilov and Larisa A. Badovskaya
Research Laboratory of Furan Chemistry, Kuban State Technological University, Moskovskaya st. 2, Krasnodar, 350072, Russian Federation; E-mail: [email protected]
Received: 1 February 2000 / Accepted: 8 February 2000 / Published: 23 February 2000

The title compound was synthesized by the reaction of N-benzyl-3-benzylamino-4-hydroxybutanamide with 3-pyridinecarboxaldehyde using the procedure described in [1]. A mixture of N-benzyl-3-benzylamino-4-hydroxybutanamide (2.98 g, 0.01 mol), 3-pyridinecarboxaldehyde (nicotinaldehyde) (1.02 g, 0.01 mol), molecular sieves (Na-A, 20 g), p-TsOH (0.02 g) and dry chloroform (50 ml) was heated to reflux for 4 h. The reaction mixture was then filtered, and the solvent was evaporated. The residue was dissolved in ethyl acetate (10 ml) and cooled to 0 °C for crystallization of desired product. The crystals obtained was separated with filtration and recrystallized from ethanol to yield 3.33 g (86 %) of 3-benzyl-4-(N-benzylcarbamoylmethyl)-2-(3-pyridyl)-1,3-oxazolidine.
M.p. 93 °C (ethanol)
IR (vaseline oil, cm-1): 3310 (N-H); 1650 (C=O); 1550 (N-H); 1605 (C=C).
1H NMR (CDCl3, 250 MHz): 2.25, 2.31 (dd, dd, 2H, CH2CO, J = 16.0 Hz); 2.41, 2.51 (d, d, 2H, NCH2, J = 14.0 Hz); 3.5 (m, 1H, NCH); 4.07, 4.26 (dd, dd, 2H, OCH2); 6.23 (broad s, 1H, NH); 7.18 (m, 11H, Ph, 5-H Py); 7.72 (dd, 1H, 6-H Py); 8.20 (dd, dd, 2H, NHCH2}, 8,50 (dd, 2H, 2,4-H Py).
Anal. calcd. for C24H25N3O2 (387.48): C 74.51; H 6.62; N 10.78; Found: C 74.39; H 6.50; N 10.85.
References:
1. Tlekhusezh, M.A.; Badovskaya, L.A.; Tyukhteneva, Z.I. Khimiya geterotsikl. Soedin. (Chemistry of Hetrocyclic compounds, Russia) 1996, 5, 711-716.
Sample availability: Available from authors and MDPI.
©2000 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/