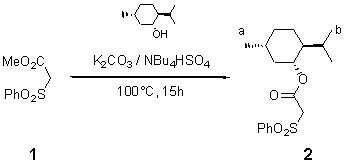
Menthyl Phenylsulfonyl Acetate
Mohammed Ramdani and Abderrahmane Elidrissi
Laboratory of Chemistry Organic-Physic, Department of Chemistry, Faculty of Sciences, University of Mohammed The First, B P 524 - 60000 Oujda, Morocco. Fax (212)6 50 06 03. E-mail: [email protected]
Received: 21 December 1999 / Accepted: 14 January 2000 / Published: 28 April 2000
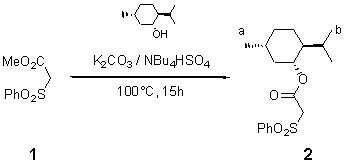
Menthyl phenylsulfonyl acetate was prepared from methyl phenylsulfonyl acetate by solid-liquid PTC trans-esterification conditions without a solvent [1]. To ester 1 (3.21 g, 15 mmol) was added (-)-menthol (3.05 g, 19.5 mmol), potassium carbonate (0.069 g, 3 mmol) and NBu4HSO4 (0.034 g, 0.6 mmol). The mixture was heated at 100 °C for 15 h in an oil bath under a dynamic vacuum, cooled to ambient temperature then treated with dilute HCl. The mixture was washed with aqueous NaCl and extracted with ethyl acetate. The product was purified by column chromatography on silica (eluent: pentane/ethyl acetate; 95/5), to give 2 (96%) as a colourless liquid.
1H NMR (CDCl3, 200 MHz): 8-7.5 (m, 5H, Ph); 4.7-4.53 (dt, 1H, CH-O); 4.1 (s, 1H, CH2C=O); 0.95-0.78 (m, 6H, J = 7.2 Hz, 2(CH3)b); 0.75-0.62 (d, 3H, J = 7.2 Hz, (CH3)a).
13C NMR (CDCl3, 50 MHz): 162 (CO2); 139, 135, 130 and 129 (Carom); 78 (CH2); 61 (CH); 31 (CH3).
IR: 1740 (CO2); 1340 and 1130 (SO2).
MS (IC-NH3, m/z): 356 (M+ + 18, 100 %).
Reference
| 1. | Loupy, A.; Sansoulet, J.; Vaziri-Zand, F. Bull. Soc. Chim. Fr. 1987, 1027-1035 and references cited therein. |
Sample Availability: Available from the authors.
© 2000 MDPI. All rights reserved.
Molecules
website www.mdpi.org/molecules/