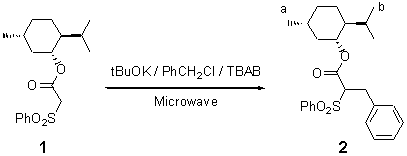
Menthyl 2-Benzyl-2-phenylsulfonyl Acetate
Mohammed Ramdani* and Abderrahmane Elidrissi
Laboratory of Chemistry Organic-Physic, Department of Chemistry, Faculty of Sciences, University of Mohammed The First, B P 524 - 60000 Oujda, Morocco. Fax (212)6 50 06 03. E-mail: [email protected]
Received: 21 December 1999 / Accepted: 14 January 2000 / Published: 29 April 1999

The product 2 was prepared from menthyl phenylsulfonyl acetate in situ by the solid-liquid PTC conditions without solvent. A mixture of ester 1 (2.5 mmol, 0.845 g), benzyl chloride (6.25 mmol, 0.79 g), tBuOK (6.25 mmol, 0.70 g) and tetrabutyl ammonium bromide (TBAB) (0.25 mmol, 0.080 g) was placed in a Pyrex tube which was then introduced into a Maxidigest MX 350 Prolabo microwave[1] monomode reactor fitted with a rotational system. An approximate final temperature (150 °C) was measured by introducing a digital thermometer at the end of the irradiation time (20 min on 150W as irradiation power). The mixture was cooled to ambient temperature. After dilution with ethyl acetate (30 ml) and subsequent filtration through Florisil, the organic product was purified by chromatography on silica (pentane:ethyl acetate, 90:10 ), to give 2 as a colourless liquid in 75% yield. No diastereoisomers were detected.
1H-NMR (CDCl3, 200 MHz): 8-7.55 (m, 5H, PhSO2); 7.3-7.1 (m, 5H, PhCH2); 4.55-4.4 (dt, 1H, CH-O); 4.3-4.18 (dd, 1H, CH-C=O); 0.95-0.78 (m, 6H, J = 7.2 Hz, 2(CH3)b); 0.75-0.62 (d, 3H, J = 7.2 Hz, (CH3)a).
13C-NMR (CDCl3, 50 MHz): 165 (ester); 135, 130 and 129 (Carom); 78 (CH); 31 (CH3).
IR: 1740 cm-1 (C=O); 1340 and 1130 (SO2)..
MS (IC-NH3, m/z): 370 (M+ + 18 - C6H4, 100 %).
Reference
| 1. | Yuliang, W.; Yaozhong, J. Synth. Commun. 1992, 22, 2287-2291. |
Sample Availability: Available from the authors.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/