A New Heterocyclization Product of Adhyperforin from Hypericum perforatum (St. John's Wort)
Suzana Vugdelija1, Vlatka Vajs2, Snezana Trifunovic1, Dejan Djokovic1 and Slobodan Milosavljevic1
1Faculty of Chemistry, Studentski
trg 16, P.O.Box 158, 11000 Belgrade Yugoslavia. Phone: 381-11-630-474.
Fax: 381-11-636-061 (E-mail: [email protected]
and E-mail: [email protected]).
2Institute for Chemistry,
Technology and Metallurgy, Njegoseva 12, 11000 Belgrade, Yugoslavia (E-mail:
[email protected])
Received: 24 February 2000 / Accepted: 4 April 2000 / Published: 30 April 2000

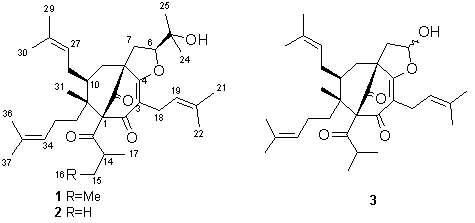
From the aerial parts of Hypericum perforatum L. we have isolated phloroglucinol 1 (see the formula), a homologue of 2, the latter isolated previously from the same extract and identified by 2D NMR (DQF COSY, PS NOESY, TOCSY, HSQC and HMBC) [1]. Compounds 1 and 2 are the heterocyclization products of adhyperforin and the well-known antibiotic hyperforin, respectively. The only difference between the 1H NMR spectra of 1 and 2 was that concerning alkanoyl side chain at C-1. Instead of two methyl doublets (0.97 and 1.08) typical for an isobutyryl group observed in the 1H NMR spectrum of 2 [1], compound 1 contained a triplet (0.78) of a methyl group (H-16) next to a methylene + a methyl doublet at 1.08 (H-17) characteristic for 2-methylbutyryl moiety. In addition, the molecular mass of 1 was higher by 14 amu.
Ethanol extraction of the air-dried ground aerial parts of H. perforatum (6.3 kg), collected at mountain Ozren (southeast Serbia) during the flowering season in July 1994, fractionation of the crude extract with supercritical CO2 at different pressures and temperatures into five fractions (F1-F5), and isolation from F2 of 2 and a degradation product 3 (with the same basic skeleton as 2, and OH instead of Me2COH at C-6), was reported previously [1,2]. Continuing this work, 10 g (out of 17.1 g) of F3 was subjected to silica gel chromatography column, starting elution with toluene and gradually increasing polarity by addition of EtOAc. Compound 1 (pale yellow viscous oil, 16 mg) was isolated from a fraction eluted with 2% (v/v) EtOAc in toluene by preparative TLC (n-hexane-EtOAc, 7.5:2.5). A fraction eluted with 3-4% (v/v) EtOAc in toluene yielded additional quantity (78 mg) of 2.
Spectroscopic data for compound 1:
1H NMR (200 MHz, CDCl3): 4.55 (dd, 5.6, 11.0, H-6); 2.66 (dd, 11.0, 13.2, H-7B); 0.78 (t, 7.3, H-16); 1.09 (d, 6.4, H-17); 3.01 (dd, 7.6, 14.5, H-18A); 3.16 (dd, 6.8, 14.5, H-18B); 5.07 (m, H-19), 1.64 (br s, H-21); 1.70 (br s, H-22); 1.22 (s, H-24); 1.39 (s, H-25); 4.92 (br t, ca 6.5, H-27); 1.70 (br s, H-29); 1.57 (br s, H-30); 1.05 (s, H-31); 5.07 (m, H-34); 1.60 (br s, H-36); 1.64 (br s, H-37).
DCI-MS (isobutane): (M+H)+ 567.
Acknowledgement: The Ministry for Science and Technology, Republic Serbia Grant.
References and Notes
| 1. | Trifunovic, S.; Vajs, V.; Macura, S.; Juranic, N.; Djarmati, Z.; Jankov, R.; Milosavljevic S. Phytochemistry 1998, 49, 1305-1310. |
| 2. | Antibiogram tests revealed a moderate activity of 3 against G+ bacteria (Micrococcus luteus and Staphylococcus aureus) and a low activity of 2 against M. luteus and no activity against S. aureus. Neither of them exhibited activity against G- bacteria (Escherichia coli) [1]. Rapid decomposition of 1 (after few days), possibly due to traces of acidic impurities, did not allow antibiogram tests. |
Sample Availability: not available.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/