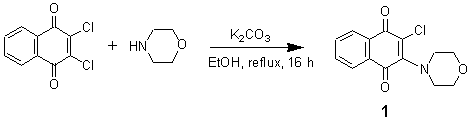
2-Chloro-3-(4-morpholino)-1,4-naphthoquinone
Sukanta Bhattacharyya*
Department of Chemistry, The University
of Mississippi, University, MS 38677
*Current Address: Argonaut Technologies,
887 Industrial Road, Suite G, San Carlos, CA 94070, USA. (E-mail: [email protected])
Received: 27 February 2000 / Accepted: 22 March 2000 / Published: 28 April 2000
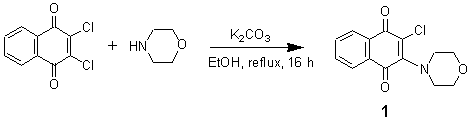
The title compound, a novel electron donor-acceptor system [1] was prepared [2] by refluxing a suspension of commercially available 2,3-dichloro-1,4-naphthoquinone in absolute ethanol and morpholine in the presence of anhydrous potassium carbonate. A mixture of 2,3-dichloro-1,4-naphthoquinone (0.90 g, 4 mmol), morpholine (0.52 ml, 6 mmol) and anhydrous potassium carbonate (0.69 g, 5 mmol) in 15 ml of absolute ethanol was refluxed for 16 h under an atmosphere of nitrogen. The mixture was then cooled to ambient temperature, 10 ml of water was added and the resulting deep violet crystals were filtered. The precipitate was washed with 1:1 ethanol and water mixture (3 x 5 ml). The crude product was further crystallized from a mixture of ethanol and dichloromethane to afford analytically pure sample of 1 as violet crystals (0.75 g, 68 %).
Mp: 150-152 oC.
IR (KBr, cm-1): 2969, 2922, 2869, 1669, 1649, 1582, 1559, 1456, 1442, 1392, 1319, 1275, 1239, 1202, 1125, 1112, 1062, 982.
1H NMR (300 MHz, CDCl3): 3.63 (t, 4H, J = 4.8 Hz), 3.87 (t, 4H, J = 4.8 Hz), 7.68-7.74 (m, 2H), 8.02 (dd, 1H, J = 8.2 and 1.8 Hz), 8.12 (dd, 1H, J = 8.2 and 1.8 Hz).
13C NMR (75.5 MHz, CDCl3): 51.8, 67.5, 126.6, 127.0, 131.3, 133.2, 134.2, 178.0, 181.8.
Anal. Calc. for C14H12NClO3: C 60.55, H 4.36, N 5.04. Found: C 60.32, H 4.49, N 5.16.
References
| 1. | De, R.; Bhattacharyya, S.; Ganguly, T. Spectrochim. Acta. Part A (Mol. Spectroscopy), 1994, 50A, 325. |
| 2. | Kutyrev, A. A.; Moskva, V. V. Russ. Chem. Rev. 1991, 60, 72. |
Sample Availability: Available from the author.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/