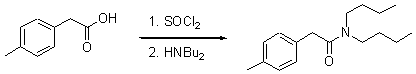
N,N-Dibutyl-2-p-tolyl-acetamide
Olivier Comte, Sylvie Genillard and Céline Nkubana
IPSC - BCH, Université de Lausanne, 1015 Lausanne, Switzerland. E-mail: [email protected]
Received: 4 April 2000 / Accepted: 6 April 2000 / Published: 28 April 2000
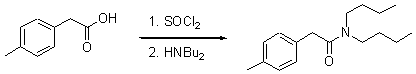
Thionyl chloride (2.18 ml, 3.56 g, 30 mmol, 1.5 eq) was added dropwise to a vigorously stirred solution of p-tolylacetic acid (3 g, 20 mmol, 1 eq) and heated to 100°C for 3 hours. After cooling to room temperature, the volatiles were removed under reduced pressure [1]. The yellow residue was dissolved in 20 ml of dry ether and dibutylamine (11.1 ml, 8.28 g, 64 mmol, 3.2 eq.) was added dropwise at 0°C. After the addition was complete, the solution was allowed to warm to room temperature and stirred for 3 hours. The crude solution was washed with 2 x 25 ml of saturated NaHCO3 solution and 2 x 25 ml of brime. The organic phase was dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by horizontal distillation (Kugelrohr) at 150°C (p=0.05 mbar) to yield 2.7 g (52 %) of the title product as a colorless oil.
IR (FT): 2958; 2872; 1642 (C=O); 1515; 1456; 1377.
1H NMR (CDCl3, 400 MHz): 7.13 (AB, J = 8.0, 2 H, Harom); 7.11 (AB, J = 8.0, 2 H, Harom); 3.64 (s, 2 H, CH2); 3.31 (m, 2 H, NCH2); 3.19 (m, 2 H, NCH2); 2.31 (s, 3 H, CH3); 1.24-1.56 (m, 8 H, 2xCH2CH2); 0.92 (t, J = 7.3, 6 H, 2xCH3).
13C NMR CDCl3, 100 MHz): 170.2 (S, C=O); 135.6 (S, Carom); 132.4 (S, Carom); 128.9 (D, J = 157, CHarom); 128.3 (D, J = 157, CHarom); 47.7 (T, J = 134, NCH2); 45.3 (T, J = 134, NCH2); 40.4 (T, J = 128, CH2CO); 30.9 (T, CH2); 29.5 (T, CH2); 20.7 (Q, CH3); 20.0 (T, CH2); 19.8 (T, CH2); 13.6 (Q, J = 126, CH3).
Mass (CI, NH3): 262 (100, [M+H]+); 156 (15, [CONBu2]+); 105 (8, [M-CONBu2]+).
Reference
1. Tietze, L. F.; Eicher, C. in: Reaktionen und Synthesen; Thieme: Stuttgart, 1981, p.92.
Sample Availability: Samples are available from MDPI.
©1999 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/