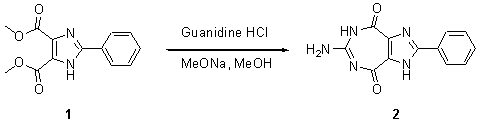
6-Amino-2-phenylimidazo[4,5-e][1,3]diazepine-4,8(1H, 5H)-dione
Huan-Ming Chen and Ramachandra S. Hosmane*
Laboratory for Drug Design and Synthesis, Department of Chemistry & Biochemistry, University of Maryland, Baltimore County, 1000 Hilltop Circle, Baltimore, MD 21250, USA. Tel. 410-455-2520, Fax 410-455-1148 (E-mail: [email protected])
Received: 17 May 2000 / Accepted: 2 June 2000 / Published: 10 July 2000
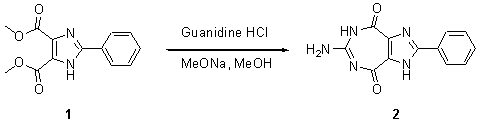
The starting imidazole diester (1) was prepared by esterification of the corresponding dicarboxylic acid acccording to the literature procedure [1,2]. A solution of dimethyl 2-phenyl-1H-imidazole-4,5-dicarboxylate (1) (0.26 g, 1 mmol) and guanidine hydrochloride (0.48 g, 5 mmol) in 50 mL of absolute methanol was stirred for 30 min at room temperature, then 2 mL of 25% sodium methoxide solution in methanol was added. The resulting mixture was stirred for 48 h at room temperature. The precipitate formed was filtered and washed with methanol to give sodium salt of the product as a white solid. The sodium salt was dissolved in water and neutralized with 2 M hydrochloric acid to yield the title compound (2) as a white solid after filtration and washing with water, yield 0.21 g (78%).
M.p. >260 oC.
TLC (CHCl3: MeOH: 30% NH4OH, 2:2:1): Rf 0.61.
1H NMR (DMSO-d6): 13.74 (br s, 1H, NH, exchangeable with D2O), 10.68 (br s, 1H, NH, exchangeable with D2O), 8.20 (m, 2H, Ph), 7.60 (br s, 1H, NH, exchangeable with D2O), 7.48 (m, 3H, Ph), 6.50 (br s, 1H, NH, exchangeable with D2O).
FABMS: 256 [MH+].
Anal. Calcd. for C12H9N5O2.3/4H2O (268.75): C, 53.63; H, 3.94; N, 26.06. Found: C, 53.62, H, 4.02; N, 26.03.
References
1. Anderson, W. K.; Bhattacharjee, D.;
Houston, D. M. J. Med. Chem. 1989, 32, 119-127.
2. Manecke, G.; Schlegel, R. Chem.
Ber. 1974, 107, 892-897.
Sample availability: available from the authors and from MDPI (MDPI ID 18548).
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/