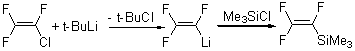
Trimethyl(trifluorovinyl)silane
Roxana M. Trifu* and George L. Gould
Department of Chemistry, University of Illinois at Chicago, Chicago, IL 60607-7061, USA
*Current address: School of Polymers and High Performance Materials, the University of Southern Mississippi, Hattiesburg, MS 39406, USA (E-mail: [email protected]
Received: 6 June 2000 / Accepted: 6 July 2000 / Published: 30 July 2000
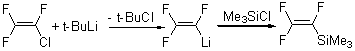
Trimethyl(trifluorovinyl)silane was prepared [1] without characterization details from bromotrifluoroethylene, which is not commercially available. The product was synthesized using several other reagents: chlorotrifluoroethylene, trifluoroethylene and various alkyl lithium compounds. An example of preparation is given here. Chlorotrifluoroethylene 3.8g (32.6 mmol) was vacuum transferred in a large storage tube over 50 ml distilled ether. t-BuLi/pentane 20 ml (32.6 mmol) was added dropwise under an argon atmosphere to the contents of the storage tube kept at -78°C. After 70 minutes addition of t-BuLi was completed and trimethylsilylchloride 3.54g (32.6 mmol) was added dropwise over a period of 1 h. The material was allowed to warm up slowly to room temperature for 7 h. Fractional distillation gave trimethyl(trifluorovinyl)silane as a colorless liquid (1.1g, 22% yield).
B.p. 65-66°C.
1H NMR (200 MHz, neat): 0.23 (s, 3H).
13C NMR (200 MHz, neat): -3.7 (s), 131.3 (ddd, J = 254.5, 67.1 and 2.8 Hz), 161.3 (ddd, J= 312.2, 271.6 and 33.6 Hz).
19F NMR (200 MHz, neat, vs. CFCl3): -88.8 (dd, J=70.9 and 25.4 Hz), -117.6 (dd, J=116.5 and 70.8 Hz), -198.6 (dd, J=116.4 and 25.2 Hz).
MS (EI, %): 154 (M+, 12), 139 ([M-CH3]+, 21), 81 (C2F3+, 100), 77 ([Si(CH3)2F]+, 50), 74 ([HSi(CH3)3]+, 45), 73 ([Si(CH3)3]+, 19), 59 (CSiF+, 41).
Reference
| 1. | Tarrant, P.; Ward, W. H. J. Org. Chem. 1966, 31, 1143-1146. |
Sample availability: sample is not available.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/