a Department of Chemistry, Shanghai Second Medical University, Shanghai200025, People's Republic of China. Tel. +86 21 63846590-464, Fax. +86 21 63842916, E-mail: [email protected]
b Department of Pharmacy, Dali Medical College, Dali, Yunnan Province-671000, People's Republic of China. Tel. +86 872 2127721, Fax. +86 872 2187801, E-mail: [email protected]
* Author to whom correspondence should be addressed.
Received: 29 August 2000 / Accepted: 19 October 2000 / Published: 25 December 2000

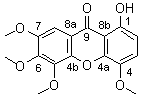
Members of genus Swertia (Gentianaceae) have been the source of many active xanthones.[1] In this note, we report a new xanthone named swertidecoraxanthone-I isolated from Swertia decora Franch. It was identified as 1-hydroxy-4,5,6,7-tetramethoxy-9H-xanthen-9-one by UV, 1H NMR, 13C NMR, MS, HMQC, HMBC and NOESY.
The air-dried, whole parts of S. decora (6.8 kg), collected in the northwestern area of Yunnan Province (China) during the later flowering season in Autumn 1998, were powdered and exhaustively extracted with 90% EtOH three times (each 24 hours) at room temperature. The combined extracts were concentrated under reduced pressure until only H2O remained. The residue then was successively extracted with gasolene, CHCl3, EtOAc and n-BuOH to yield four corresponding fractions. The CHCl3 extract (28.5 g) was subjected to a silica gel column and eluted with a gradient of CHCl3-Et2O, collecting 12 fractions of 100 ml each. The fractions of 1-4 were combined according to TLC examination and chromatographed repeatedly over silica gel on elution with petroleum ether-CHCl3-Et2O, collecting 12 fractions. 6 parts were combined according to TLC to afford swertidecoraxanthone-I (300 mg) as a yellowish orange rectangular lumps.
Mp: 163-165°C.
1H NMR (400 MHz, CDCl3): 12.534 (1H, s, 1-OH), 7.184 (1H, d, J = 8.9 Hz, 3-H), 6.689 (1H, d, J = 8.9 Hz, 2-H), 6.834 (1H, s, 8-H), 3.951 (3H, s, 4-OMe), 4.033 (3H, s, 5-OMe), 3.917 (3H, s, 6-OMe), 3.984 (3H, s, 7-OMe).
13C NMR (100 MHz, CDCl3): 154.902 (C1), 108.915 (C2), 119.303 (C3), 139.382 (C4), 153.398 (C5), 139.884 (C6), 159.657 (C7), 96.354 (C8), 181.221 (C9), 144.947 (C4a), 154.515 (C4b), 109.407 (C8a), 109.451 (C8b), 57.346 (4-OMe), 62.093 (5-OMe), 61.510 (6-OMe), 56.506 (7-OMe).
IR (cm-1, KBr): 3425.0(br), 2941.0, 1650.8, 1602.6, 1479.2, 1427.1, 1328.7, 1282.5, 1236.2, 1101.2, 972.0, 815.8.
UV (CH3OH): 209.8, 236.4, 255.8 (sh), 280.4, 302.4 (sh), 381.6.
EI-MS: 333 (13.50), 332 ([M+], 61.79), 318 (20.42), 317 (100.00), 302 (17.77), 299 (17.31), 287 (16.67), 259 (12.64).
Acknowledgement: This work is supported by Shanghai Municipal Educational Committee (grants S970204).
Reference
1. Xiao H.; Lu Y.; Chen Z. N.; Qian J. F. Advances in Chemical and Pharmacological Studies on Medicinal Plants of Swertia. Chinese Traditional and Herbal Drugs. 1999, 30 (suppl.), 36-39.
Sample availability : Available from the authors and MDPI.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/