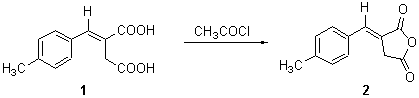
E-(4-Methylbenzylidene)succinic anhydride
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King abdul-Aziz University, Jeddah 21589, P. B. Box 80203, Saudia Arabia. Tel. (+696)-2-6952293, Fax (+696)-2-6952292, E-mail: [email protected]
Received: 26 September 2000 / Accepted: 16 November 2000 / Published: 25 December 2000
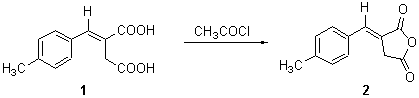
The title compound was obtained by dehydration of the corresponding diacid 1 (12.1 g, 55 mmol) by boiling in acetyl chloride (50 ml) for four hours. The excess acetyl chloride was removed under vacuum and the residue crystallised from ethanol to give the anhydride 2 as yellow crystals (5.1 g, 46%).
M.p. 210-212°C (EtOH, Uncorrected).
UV (EtOH) (e dm3.mol-1.cm-1): 291 (25000), 396 (5000).
IR (KBr, cm-1): 1805 (C=O), 1751 (C=O).
1H-NMR (400 MHz, CDCl3): 7.85 (1H, s, HC=), 7.28 (2H, d, J 7.8 Hz), 7.19 (2H, d, J 7.8 Hz), 3.51 (2H, s, CH2), 2.37 (3H, s, CH3).
13C-NMR (100 MHz, CDCl3): 21.3 (CH3), 33.71 (CH2), 129.2, 129.3, 132.3, 135.0, 141.5 (CH=C), 169.8 (C=O), 173.4 (C=O).
Anal. Calc. For C12H10O3 (202.12): C, 71.30, H, 4.95; found : C, 71.11, H, 5.12.
References
| 1. | Stobbe, H. Ber. 1904, 37, 2236. |
| 2. | Johnson, W. S.; Duab, G. H. Org. React. 1951, 6, 1. |
Sample availability: available from the authors and MDPI.
©2000 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/