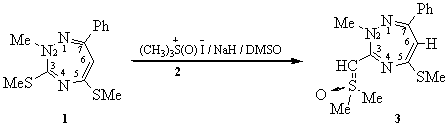
2-Methyl-5-methylmercapto-3-dimethylsulfoxymethine-7-phenyl-1,2,4-triazepine
My. Y. Ait Itto1, A Hasnaoui1, A. Riahi2 and A. Huet3
1Laboratoire de chimie des substances
naturelles et des Hétérocycles, Département de Chimie,
Faculté des Sciences Semlalia, B.P. 2390, 40001 Marrakech-Maroc.
2Université de Reims,
UFR Sciences, UMR N° 6519, Réactions Sélectives et Applications,
B.P. 1039, 51687 Reims Cedex 2 France. 3Laboratoire de Synthèse
Organique, Université de Maine, Le Mans-France. *Phone: 2124434349,
Fax: 2124437408, E-mail: [email protected].
Received: 20 September 2000 / Accepted: 16 November 2000 / Published: 25 December 2000
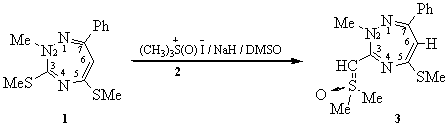
All the following operations were performed under an inert atmosphere using standard vacuum line techniques. Trimethyloxosulfonium iodide (2, 0.73 g, 3.3 mmol) [1] was suspended in anhydrous DMSO (6 mL) and sodium hydride (80 % suspension in mineral oil, 0.1 g, 3.3 mmol) was added at room temperature. The mixture was vigorously stirred for 15 min and then cooled to 10°C before adding rapidly a solution of the 1,2,4-triazepine 1 (0.3 g, 1.1 mmol) [2] in anhydrous DMSO (3 mL). After stirring at room temperature during 48 hours, the reaction mixture was poured in cold water (40 mL) and extracted with chloroform (3 x 15 mL). The combined organic layers were washed with water (3 x 15 mL), dried over anhydrous MgSO4. The solvent was then evaporated under vacuum. The pure product 3 was obtained as an orange solid after recristallization from carbon tetrachloride (85 % yield).
Mp.: 169-170°C
1H NMR (CDCl3, 400 MHz): 2.35 (s, 3H, SCH3); 3.11 (s, 3H, NCH3); 3.47 (s, 6H, (CH3)2SO); 3.71 (s, 1H, H-C=S); 5.66 (s, 1H, H-C6); 7.31-7.70 (m, 5H, aromatic CH).
13C NMR (CDCl3, 100 MHz, assignment based on NOESY, HMBC and COSY experiments): 14.82 (SCH3); 41.42 (NCH3); 42.43 ((CH3)2SO); 59.79 (H-C=S); 100.95 (C6); 127.98; 128.02; 129.39; 136.85 (aromatic C-H); 161.59 (C3); 163.18 (C7); 164.76 (C6).
MS-EI+(m/z, %): 321 (100, [M]+), 242 (30, [M-(CH3)2SO]+), 230 (5, [M- CH3)2SOCH]+).
Anal calcd. for C15H19N3S2O (321.46): C, 56.04; H,5.96; N, 13.07; found C, 55.91; H, 6.04; N, 13.02 %.
References
| 1. | Corey, E. J.; Chakovsky, M. J. Am. Chem. Soc. 1965, 87, 1353. |
| 2. | Ait Itto, My. Y.; Hasnaoui, A.; Riahi, A.; Lavergne, J.-P. Tetrahedron Lett. 1997, 38, 2087. |
Sample availability: available from the authors and MDPI.
©2000 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/