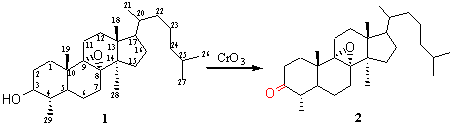
8a,9a-Epoxy Nor-31-lanosten-3-one
M. Daoubi1*, A. Benharref1, E. Kossareva2 and M. Pierrot2
1 Laboratoire de Chimie des
Substances Naturelles et Hétérocycles, Université
Cadi Ayyad, Faculté des Sciences Semlalia, B.P : 2390, Marrakech.
Maroc.
E-mail: [email protected].
2 LBS-UMR 6517, Centre Scientifique Saint-jérôme, 13397 Marseille, CEDEX 20, France.
Received: 29 October 2000 / Accepted: 30 November 2000 / Published: 25 December 2000

To the epoxide 1 (1 g, 2.32 mmol) in acetone (50 ml) [1] was added dropwise at 0°C a solution of CrO3 (0.7 g, 7 mmol) in acetone (20 ml). After stirring for 2 hours, the solvent was removed in vacum. Water was added and the product was extracted by 3 times CH2Cl2. The organic layer was dried (Na2SO4), concentrated and purified by chromatography on silica gel column using hexane/EtOAc 93/7 as solvent to give 2 (0.73 g, 72 %).
Mp : 153-154 °C.
IR : 1740 cm-1.
MS (EI, 70eV) : 428.7 (M+.).
1H NMR (200 MHz, CDCl3) : 0.74 (s, C18-H3); 0.82 (s, C19-H3); 0.79 (d, J=6 Hz, C21-H3); 0.77 (d, J=6 Hz, C26 and C27-H6); 1.22 (s, C28-H3); 0.80 (d, J=6 Hz, C29-H3).
13C NMR (50 MHz, CDCl3) : 33.13 (C1); 38.44 (C2); 211.10 (C3); 47.31 (C4); 44.06 (C5); 21.50 (C6); 27.44 (C7); 68.27 (C8); 66.93 (C9); 47.73 (C10); 22.04 (C11); 30.90 (C12); 42.61 (C13); 36.38 (C14); 35.3 (C15); 35.13 (C16); 41.95 (C17); 10.55 (C18); 18.89 (C19); 25.85 (C20); 19.81 (C21); 36.59 (C22); 23.03 (C23); 26.95 (C24); 36.22 (C25); 15.16 (C26); 14.51 (C27); 21.51 (C28); 21.80 (C29).
The structure of the compound 2 were established by X-ray crystal structure determination.
Reference
[1] Smissman, E. E.; Pengman, E.; Greese, M. W. J. Org. Chem. 1970, 64, 1352-1356.
Sample availability : Available from the authors and MDPI.
© 2000 MDPI. All rights reserved.
Molecules
website www.mdpi.org/molecules/