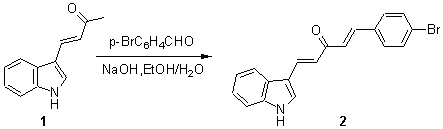
E,E-1-(4-Bromophenyl)-5-(3-indolyl)-1,4-pentadien-3-one
Gang-Chun Sun1, Rui-Yun Guo2, Jian-Qiang Qu1, Liu-Fang Wang1*,Jun-Biao Chang2* and Rong-Feng Chen2
1National Key Laboratory of
Applied Chemistry, Lanzhou University, Lanzhou 730000, P.R.China.
2Henan Institute of Chemistry,
Zhengzhou 450002, P.R.China. (E-mail: [email protected])
Received: 20 September 2000 / Accepted: 30 November 2000 / Published: 25 December 2000
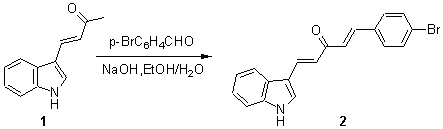
3-(1-Butenone-3)indole 1 was prepared by previously published method [1]. The solution of 1 (93mg, 0.5mmol), p-bromobenzaldehyde (93mg, 0.5mmol), sodium hydroxide (100mg) in a mixture of ethanol (2ml) and water (2ml) was stirred at room temperature for 24 hours. The precipitate was then filtered and washed with water for several times. The crude product was purified by recrystalisation from acetone to give a yellow powder of E,E-1-(4-bromophenyl)-5-(3-indolyl)-1,4-pentadien-3-one (106mg, 60%).
Mp: 259-260ºC.
IR (KBr, cm-1):3174.6 (N-H), 1643.2 (C=O), 1612.4 (C=C), 1569.9, 1515.9, 1488.9, 1434.9, 1346.2, 1195.8, 1130.2, 964.3, 848.6, 744.5.
UV-Vis (nm): 215.5, 311.5, 465.0.
1H NMR (400 MHz, DMSO-d6): 7.14 (d, J=16Hz, 1H, 4-H); 7.19-7.27 (m, 2H, 5'-H, 6'-H); 7.47 (d, J=16Hz, 1H, 2-H); 7.47-7.52 (m, 1H, 7'-H); 7.63 (d, J=16Hz, 1H, 5-H), 7.66 (d, J=8.4Hz, 2H, 3"-H, 5"-H); 7.76 (d, J=8.4Hz, 2H,2''-H, 6''-H); 8.05 (d, J=16Hz, 1H, 1-H); 8.02 (d, J=2.8Hz, 1H, 2'-H); 8.05-8.09 (m, 1H, 4'-H), 11.88 (s, 1H, 1'-H).
MS m/z: 352 (M+).
Acknowledgement: This research was supported by the NSF of China (NO:29972009) and the NSF of Gansu Province (NO:ZS991-A23-059-Y).
References
| 1. | Xie, J.; Xie, L.; Gu, Z.; Liu, Y.; Wang, Z. Acta Pharmaceutica Sinica 1994, 23, 732- 736. |
Sample availability: not available.
©2000 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/