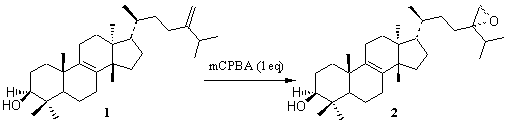
1 Laboratoire de Chimie des
Substances Naturelles et des Hétérocycles, Université
Cadi Ayyad, Faculté des Sciences Semlalia. BP 2390 Boulevard Prince
My Abdellah. Marrakech, Maroc. E-mail: [email protected];
E-mail: [email protected]
2 Laboratoire de Chimie de
Synthèses et Activations de Biomolécules, Ecole Nationale
Supérieure de Chimie de Rennes. Avenue du Général
Leclerc 35700 RENNES, France.
Received: 31 August 2000 / Accepted: 19 October 2000 / Published: 25 December 2000
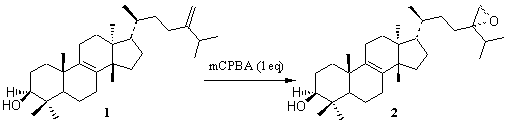
To a solution of 1g (2.27 mmol) of 1 in 40ml of chloroform at room temperature was added 0.55g (2.27 mmol) of meta-chloroperbenzoic acid (mCPBA). The solution was stirred for 3 hours and then it was washed with 10% aqueous sodium bisulphite and saturated sodium bicarbonate, dried over sodium sulphate and concentrated under reduced pressure to give 0.89g (85.85%) of the epoxide 2 after purification by flash chromatography (10% ethyl acetate in hexane).
Mp: 139-140 (hexane).
MS: 442 ([M]+).
IR (KBr): 3400 (OH); 1638 (C=C); 1287 (C-O epoxide).
1H NMR (200 MHz, CDCl3): 3.2 (dd, J1=12 Hz, J2=4 Hz, C3-H); 2.55 (m, C31-H2); 0.74 (s, C18-H3); 0.87 (s, C19-H3); 0.79 (s, C28-H3); 0.97 (d , J=2 Hz, C26-H3); 0.99 (d, J=2Hz, C27-H3); 0.93 (d, J=6Hz, C21-H3); 0.94 (s, C29-H3); 0.95 (s, C30-H3).
13C NMR (100 MHz, CDCl3) (ppm): 78.48 (C3); 133.62 (C8); 133.0 (C9); 68 (C24); 62.3 (C31).
Reference
| 1. | Benharref, A.; Lavergne, J.-P. Bull. Soc. Chim. Fr. 1985, 965. |
Sample Availability: Available from the authors and from MDPI.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/