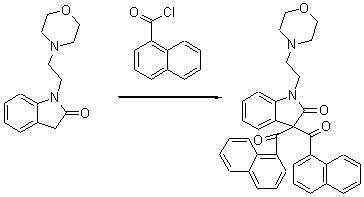
N-(2-Ethylmorpholino)-3-bis-keto(1-naphthyl)oxindole
Gerard P. Moloney
Department of Medicinal Chemistry, Victorian College of Pharmacy (Monash University), 381 Royal Parade Parkville, Victoria 3052, Australia, E-mail: [email protected]
Received: 4 September 2000 / Accepted: 29 September 2000 / Published: 25 March 2001
As part of a research programme targeting novel indole-like molecules as potential cannabinoid agonists [1-4] we synthesised N-(2-ethylmorpholino)-3-bis-keto(1-naphthyl)oxindole.

N-(ethylmorpholine) oxindole (409.0 mg, 1.66 mmol) was dissolved in anhydrous THF (25.0 mL) and the reaction mixture was cooled to -78 °C then HMPA (868.0 mL, 4.99 mmol) was added followed by KHMDS (3.66 mL, 1.83 mmol) being added dropwise. The solution was stirred for 30 minutes at -22 °C then cooled to -78 °C after which 1-naphthyl chloride (475.0 mg, 2.49 mmol) was added. The reaction was allowed to stir up to room temperature overnight. The solvent was evaporated under reduced pressure and water was added and the aqueous phase was extracted with ethyl acetate, dried over magnesium sulphate, filtered, and evaporated under reduced pressure to afford (552.5 mg, 60.0 %) of the undesired N-(ethylmorpholine)-3-bis keto(-1-naphthyl) oxindole as a yellow powder.
M.p. 80-85 °C.
MS: 555 (M+1)+..
IR: 3080, 1800, 1600, 1420, 1300, 1110, 1100, 950, 700.
1H NMR (300 MHz, CDCl3): 2.55 (m, 4H, 2 x CH2), 2.66 (t, J = 6.9 Hz, 2H, CH2), 3.71 (m, 4H, 2 x CH2) 3.93 (t, J = 6.9 Hz, 2H, CH2), 6.07 (m, 1H, ArH), 6.59 (dd, J = 0.9 Hz, J = 7.5 Hz, 1H, ArH), 6.86 (d, J = 7.8 Hz, 1H, ArH), 7.15 (m, 1H, ArH), 7.55 (m, 5H, 5 x ArH), 7.85-8.13 (m, 6H, 6 x ArH), 8.28 (m, 1H, ArH), 8.53 (m, 1H, ArH), 8.95 (m, 1H, ArH).
Anal. calcd. for C36H30N2O4 C 77.96, 5.45: Found C 77.65, H 5.50.
References
| 1. | Ward, S. J.; Mastriani, D.; Casiano, F.; Arnold, R. J. Pharmacol. Exp. 1990, 255, 1230-1239. |
| 2. | Bell, M. R.; D'Ambra, T. E.; Kumar, V.; Eissenstat, M. A.; Herrmann, J. L.; Wetzel, J. R.; Rosi, D.; Philion, R. E.; Daum, S. J.; Hlasta, D. J.; Kullnig, R. K.; Ackerman, J. H.; Haubrich, D. R.; Luttinger, D. A.; Baizman, E. R.; Miller, M. S.; Ward, S. J. J. Med. Chem. 1991, 34, 1099-1110. |
| 3. | Ward, S. J.; Miller, M. S.; Luttinger, D. A.; Eissenstat, M. A.; Bell, M. R. Neurosci. Abstr. 1988, 14, 324. |
| 4. | D'Ambra, T. E.; Estep, K. G.; Bell, M. R.; Eissenstat, M. A.; Josef, K. A.; Ward, S. J.; Haycock, D. A.; Baizman, E. R.; Casiano, F. M.; Belgin, N. C.; Chippari, S. M.; Grego, J. D.; Kullnig, R. K.; Daley, G. T. J. Med. Chem. 1992, 35, 124-135. |
Sample availability: available from the authors and MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/