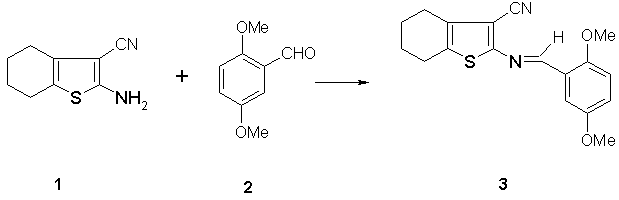
3-Cyano-2-(2,5-dimethoxybenzalamino)-4-5-tetramethylenethiophene
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia. Tel.(+696)-2-6952293, Fax (+696)-2-6952293, E-mail: [email protected]
Received: 18 December 2000 / Accepted: 15 May 2001 / Published: 25 May 2001

3-Cyano-2-(2,5-dimethoxybenzalamino)-4-5-tetramethylenethiophene was prepared by condensation of 2-amino-3-cyano-4,5-tetramethylenethiophene 1 and 2,5-dimethoxybenzaldehyde 2 [1,2]. A mixture of 2-amino-3-cyano-4,5-tetramethylenethiophene 1 (1.0 g, 5.62 mmol) and 2,5-dimethoxybenzaldehyde 2 (0.93 g, 5.62 mmol) in ethanol (20 mL) was heated under reflux for four hours. The reaction mixture was left to cool to room temperature and orang crystals was separated, filtered and washed with ethanol. Rerecrystallization from ethanol gave 3 as orange crystals (1.74 g, 95%).
Mp. 140-142 oC (EtOH, uncorrected).
UV lmax (nm; Acetone)/e (dm3.mol-1.cm-1): 408/11410.
IR (KBr) 2220 (CN), 1600 (C=N).
1H-NMR (400 MHz; CDCl3; Me4Si): 1.85 (4H, d, 2xCH2-5,6), 2.67 (4H, dd, 42xCH2-4,7), 3.84, 3.85 (6H, s, CH3O), 6.85 (1H, d, J = 9.0 Hz), 7.01 (1H, dd, J46 = 3.1 Hz, J 43 = 9.0 Hz, H-4), 7.72 (1H, d, J64 = 3.1 Hz, H-6), 8.82 (1H, s, CH=N-).
13C-NMR: 22.0, 23.1, 24.3, 25.2, 55.8, 56.1 (2xCH3O), 106.4 (CN), 110.5, 112.6, 114.6, 121.3, 123.9, 132.1, 134.9, 153.7, 154.7, 154.8 (CH=N), 160.9.
Anal.Calc. for C18H18N2O2S (326.420): C 66.23, H 5.56, N 8.58; found : C 66.41, H 5.35 , N 8.39.
References
1. Asiri, A. M. Pak. J. Sci. Ind. Res.
1998,
41,
242.
2. Chalkley, L. Chem. Rev. 1929,
6,
217.
Sample availability: available from the authors and MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/