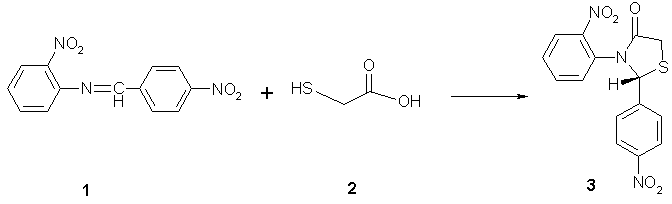
3-(2-Nitrophenyl)-2-(4-nitrophenyl)-4-thiazolidinone
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia. Tel.(+696)-2-6952293, Fax (+696)-2-6952293, E-mail: [email protected]
Received: 18 December 2000 / Accepted:
15 May 2001 / Published: 25 May 2001

The 4-thiazolidinone 3 was prepared from the Schiff base 1 thioglycolic acid 2 [1,2]. A mixture of 4-(Nitrobenzalamino)-(2-nitrobenzen) 1 (3.0 g, 0.011 mmol) and thioglycolic acid 2 (1.02 g, 0.011 mol) was refluxed for 15 hours at 110-120 oC in an oil bath. Sodium bicarbonate solution was added for removal of excess thioglycolic acid. The product was isolated and recrystallized from ethanol as orange crystals (3.52 g, 92%).
Mp. 70-72 °C (EtOH, uncorrected).
UV lmax (nm; Acetone)/e (dm3.mol-1.cm-1) 280/3185, 401/6107.
IR (KBr) 1690 (C=O), 700 (C-S-C).
1H-NMR (400 MHz; CDCL3; Me4Si) dH3.09 (2H, CH2), 6.07 (1H, d, S-CH), 6.71 (2H, dd, J = 7.6 Hz), 6.81 (2H, d, J = 8.3 Hz), 7.36 (2H, d, J = 7.33 Hz), 8.11 (2H, d, J = 8.5 Hz).
13C NMR (100 MHz; CDCl3):: 20.0 (CH2), 51.8 (C-S), 111.83, 116.1, 116.97, 118.75, 126.22, 135.65, 144.65, 196.24.
Anal.Calc. for C15H11N3O5S (345.339): C 52.17, H 3.21, N 12.17; found : C 52.33, H 3.11 , N 11.98.
References
1. Patoli, V. N.; Patel, P. K.; Xi, A. J. J. Indian Chem. Soc. 1994,
71,
683.
2. Ergenc, N.; Capan, G.; Guenay, N. S.; Oezkirimli, S.; Geuengoer,
M.; Oezbey, S.; Kendi, E. Archi der Pharm. 1999, 332,
343.
Sample availability: available from the authors and MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/