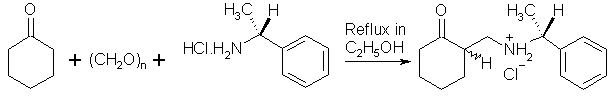
(+)-(1R)-N1-{[(1R,1S)-2-Oxocyclohexyl]methyl}-1-phenyl-1-ethanaminium Chloride
Edmont V. Stoyanov* and Ivo C. Ivanov
Faculty of Pharmacy, Medical University of Sofia, Dunav 2, BG-1000 Sofia,
Bulgaria
Tel. (+359 2) 988 3142; Fax (+359 2) 987 9874; E-mail: [email protected]
Received: 20 January 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
Keywords: Mannich base, cyclohexanone, aminoketones, aminomethylation,
primary amine

The Mannich bases of cyclohexanone with benzylamine and 3,4-methylenedioxybenzylamine hydrochlorides have been prepared in moderate yields using aqueous formaldehyde solution [1]. We report now the synthesis of an analogous product from (R)-(+)-1-phenylethylamine. A mixture of cyclohexanone (2.00 g, 0.02 mol), paraformaldehyde (1.20 g, 0.04 mol) and (+)-(R)-1-phenylethylamine hydrochloride (3.18 g, 0.02 mol) was refluxed under stirring in anhydrous ethanol (15 ml) for 5 h (TLC monitoring). The reaction mixture gradually turned into a solution. The solvent was then removed under reduced pressure and the residue was triturated with ice-cooled acetone (20 ml). The separated crystals were filtered, washed with cold acetone, recrystallized from n-butanol and air-dried. Yield: 3.45 g (60 %) of the title compound as colorless crystals. TLC homogeneous, optically active mixture of (R,R)- and (R,S)-diastereomers (TLC: silica gel Merck GF254 Al-sheets, eluted by chloroform-ethanol 3:1).
Mp. 175-176 ºC (n-butanol); [a]D = +10.0° (c = 1.00, CH3OH).
1H NMR (300 MHz, d6-DMSO): 1.12-1.30 (m, 1H), 1.40-1.60 (m, 2H), 1.62 (d, J = 6.7 Hz, CH3), 1.65-1.79 (m, 1H), 1.87-2.03 (m, 1H), 2.12-2.46 (m, 4H), 2.85-3.00 (m, 1H), 3.00-3.13 (m, 1H), 4.36 (m,1H, CH3CH), 7.33-7.47 (m, 3Harom.), 7.60-7.69 (m, 2Harom.), 9.46 and 9.67 (br. d, N+H2).
FT IR (neat): 3100-3500, 3030-3080, 3027, 2930, 2859, 1705 (C=O), 1493, 1449, 1368, 1352, 1312, 1196, 1127, 1076, 762, 702.
FAB MS [glycerol; m/z (%)]: 499 (6; 2M + 2H+ + Cl–), 232 (100; MH+ = C15H22NO+), 155 (5), 134 (5), 105 (25), 55 (3).
Anal. calcd. for C15H22NOCl (267.80): C 67.28, H 8.28, N 5.23, Cl 13.24; found C 66.97, H 8.22 , N 5.23, Cl 13.46.
Reference
1. Mannich C.; Hieronimus O. Ber. Dtsch. Chem. Ges. 1942, 75, 49-53.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/