Faculty of Pharmacy, Medical University of Sofia, Dunav 2, BG-1000 Sofia,
Bulgaria
Tel.: (+359 2) 981 7025; Fax: (+359 2) 987 9874; E-mail: [email protected]
Received: 23 February 2001 / Accepted:
15 May 2001 / Published: 25 May 2001

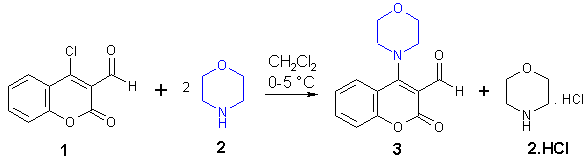
The title compound 3 has been allegedly prepared earlier [1]
by refluxing the same starting materials 1 and 2 in ethanol
for 10 min. but no characteristic data of the product has been reported
till now except its melting point. Our attempts to reproduce the procedure
given in [1] failed: a complex mixture of unidentified products resulted
(TLC-monitoring). The aldehyde 1 was prepared according to [2]).
A solution of morpholine (2; 1.75 g, 20 mmol)
in 10 ml of dichloromethane was gradually added under stirring to an ice-cooled
mixture of 4-chloro-2-oxo-2H-chromene-3-carbaldehyde (1; 2.09 g, 10 mmol)
in 25 ml of dichloromethane. After stirring for 30 min. at 0-5 °C the
mixture was washed with 3x10 ml of water in order to remove unreacted morpholine
and its salt. The organic phase was dried over MgSO4 and the solvent was
evaporated under reduced pressure. The dry, flake-like residue was recrystallized
from 1,4-dioxane. Yield: 1.44 g (56%) of 3 as yellow crystals, m.p. 162-164
°C, TLC homogeneous (TLC control: silica gel pre-coated Al-sheets Merck
GF254, eluted by chloroform-acetone 3:2).Mp.
162-164 °C. After twofold recrystallization from
dioxane: yield 1.00 g (39%), yellow crystals.
Mp. 166-168 °C (Lit. [1] m. p. 165 °C).
1H NMR (100 MHz; CDCl3): 3.4-3.8 [m, 4H, -N(CH2)2], 3.8-4.1 [m, 4H, O(CH2)2], 7.1-8.0 (m, 4Harom.), 10.2 (s, CHO).
FT IR (cm-1; nujol): 1699 (C=O, aldehyde), 1674 (C=O, lactone), 1607, 1590, 1526, 1302, 1286, 1111, 961, 916, 777, 758; (fluorolube): 2855-2950, 1701 (C=O, aldehyde), 1674 (C=O, lactone), 1607, 1590, 1526, 1428.
EI-MS [70 eV; m/z (%)]: 259 (M.+; 82), 242 (100), 230 (27), 212 (45), 202 (34), 186 (13), 174 (43), 161 (14), 146 (93), 118 (27), 89 (36), 63 (20), 28 (75).
Anal. calcd. for C14H13NO4 (259.26): C 64.86, H 5.05, N 5.40; Found C 64.83, H 5.10, N 5.34.
References
1. Moorty S.R.; Sundaramurthy V.; Subba Rao N. V. Indian J. Chem.
1973,
11,
854-856.
2. Heber D.; Ivanov I. C.; Karagiosov S. K. J. Heterocycl. Chem.
1995,
32,
505-509.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved.