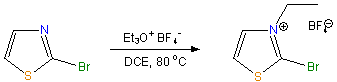
2-Bromo-3-ethylthiazolium Tetrafluoroborate (BET)
Györgyi Kovács 1*, Zoltán Kele 1, Péter Forgó 2 and Lajos Kovács 1
1. Department of Medicinal Chemistry, University
of Szeged, Dóm tér 8, H-6720 Szeged, Hungary. Phone: +36
62 54 51 45, Fax: +36 62 54 59 71, *E-mail: [email protected]
2. Department of Organic Chemistry , University
of Szeged, Dóm tér 8, H-6720 Szeged, Hungary
Received: 20 January 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
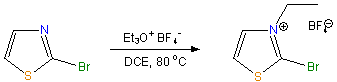
Recent progress in peptide synthesis resulted
in the elaboration of novel coupling agents. One successful approach involves
the application of thiazolium salts, e.g. 2-bromo-3-ethyl-4-methylthiazolium
tetrafluoroborate (BEMT) [1-3]. This compound can be prepared from 2-bromo-3-ethyl-4-methylthiazole
[1, 2]. We have found that a simpler analogue, 2-bromo-3-ethylthiazolium
tetrafluoroborate (BET), can be prepared in convenient steps from the commercially
available 2-aminothiazole using the procedure by Dondoni et al.
[4] to obtain 2-bromothiazole which, in turn, was readily transformed into
the title compound. In our experience BET is a highly efficient coupling
reagent in the synthesis of peptide nucleic acid (PNA) oligomers in solution
phase.
To a stirred solution of 2-bromothiazole [4]
(1.78 mL, 20.0 mmol) in 1,2-dichloroethane (DCE, 20 mL) the solution of
triethyloxonium tetrafluoroborate (11.4 g, 60.0 mmol) in DCE(60 mL) was
added over 45 min. at 80 °C. When the reaction was complete (16 h)
according to TLC (n-buthanol : acetic acid : water = 4:1:1) the solution
was concentrated
in vacuo. After the residue was precipitated from
diethyl ether it was recrystallized from abs. acetonitrile/ethyl
acetate to afford white plates. Yield: 4.48 g (80 %).
Mp: 137.9-139.2 °C.
1H NMR (DMSO-d6, 500 MHz, d, ppm): 1.47 (t, J = 7.0 Hz, 3H, CH2CH3); 4.52 (q, J = 7.0 Hz, 2H, CH2CH3); 8.35 (d, J = 4.1 Hz, 1H, aryl); 8.56 (d, J = 4.1 Hz, 1H, aryl).
13C NMR (DMSO-d6, 125 MHz, d, ppm): 14.19 (CH2CH3); 50.61 (CH2CH3); 127.45 (C-5); 137.73 (C-4); 146.50 (C-2).
ESI-MS (m/z, %): 191.8 (100, [C5H779BrNS]+); 193.8 (97, [C5H781BrNS]+).
Anal. cald. for C5H7BBrF4NS (279.891): C, 21.45; H, 2.52; Br, 28.55; F, 27.15; N, 5.00; S, 11.46; found C, 21.47; H, 2.51; Br, 28.52; F, 27.20; N, 5.01; S, 11.44.
References
1. Li, P.; Xu, J. C. Tetrahedron Lett.1999,
40,
8301-8304.
2. Li, P.; Xu, J. C. J. Org. Chem. 2000,
65,
2951-2958.
3. Li, P.; Xu, J. C. Tetrahedron 2000,
56,
8119-8131.
4. Dondoni, A. in Modern synthetic methods;
Scheffold, R., Ed.; Verlag Helvetica Chimica Acta & Verlag Chemie:
Basel, Weinheim, New York, Cambridge, 1992; Vol. 6, p 385.
Sample availability: sample available from the authors and MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website www.mdpi.org/molecules/