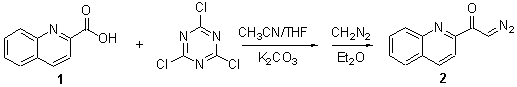
1-Diazo-2-(2-quinolinyl)-2-ethanone
David C. Forbes,* Charles O. Gaston, Derrick L. Lewis, Doug W. Morrison and Mary C. Smith
Department of Chemistry, University of South Alabama, Mobile, Alabama 36608-0002 USA, Email: [email protected]
Received: 20 January 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
Keywords: diazoketone, cyanuric chloride, diazomethane, diazo transfer
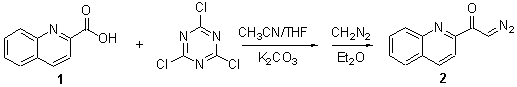
Diazoketone 2 was prepared using 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride) as coupling reagent allowing for the efficient transfer of diazomethane to commercially available carboxylic acid 1 [1]. To a 125 mL Erlenmeyer flask containing a large magnetic stir bar was added, in the following order, potassium carbonate (2.1 g, 12.9 mmol, 2.0 equiv), CH3CN (20 mL) and cyanuric chloride (0.47 g, 2.6 mmol, 0.4 equiv). The flask was cooled externally with an ice bath (0 °C) and after approximately 5 min a solution of THF (20 mL) containing the carboxylic acid 1 (1.1 g, 6.4 mmol, 1.0 equiv) was added via syringe pump over a period of 1 h. The reaction mixture was kept at 0 °C for a period of 2 h at which time the contents of the reaction mixture slowly turned into a milky-white slurry. The reaction mixture was allowed to stir for an additional 45 min at which time CH2N2 (17.4 mmol, 2.7 equiv) as a cold (0°C) diethyl ether solution (50 mL) was added in three equal portions.The ice bath containing the reaction mixture was packed with ice and allowed to slowly warm to room temperature as it stirred overnight (approx. 12 h). The crude reaction mixture was transferred to a larger flask (approx. 250 mL) and diluted with diethyl ether (total volume approx. 200 mL). The yellow solution was filtered and concentrated in vacuo. The viscous oil was then immediately purified via silica gel chromatography [hexane/EtOAc, 8/1, 30 ´ 60 mm SiO2, 10 mL fractions]. Recrystallization with hexane (8 mL) afforded analytically pure diazoketone 2 in 737 mg (3.7 mmol, 58% yield) as a yellow solid.
Mp. 44-50°C.
1H NMR (300 MHz, CDCl3): 8.19 (d, J = 8.5, 1H), 8.11 (d, J = 8.5, 1H), 8.04 (d, J = 8.2, 1H), 7.72 (d, J = 8.2, 1H), 7.69 (ddd, J = 8.5, 6.9, 1.4, 1H), 7.55 (ddd, J = 8.2, 7.1, 1.4, 1H), 6.90 (s, 1H).
13C NMR (75.5 MHz, CDCl3): 186.35, 152.00, 146.94, 137.20, 130.19, 130.13, 129.87, 128.38, 127.76, 117.42, 53.48.
IR (thin film): 2108 (C=N2); 1618 (C=O).
TLC Rf 0.51 (hexane/EtOAc, 4/1).
HRMS (FAB) calcd. for C11H7N3O 198.06674, found: m/z 198.069351.
Acknowledgment: We are grateful to the donors of the Petroleum Research Fund, administered by the American Chemical Society, the Department of Chemistry and Research Council at the University of South Alabama for support of this research.
Reference
1. Forbes, D. C.; Barrett, E. J.; Lewis, D. L.; Smith, M. C. Tetrahedron Lett. 2000, 41, 9943.
Sample Availability: Available from the authors.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website www.mdpi.org/molecules/