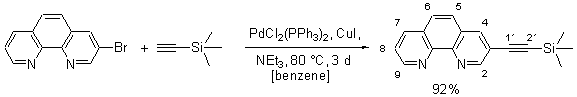
3-Trimethylsilanylethynyl-[1,10]phenanthroline
Christoph Michel, Davood Habibi and Michael Schmittel*
FB 8 - OC 1 (Chemie - Biologie), Universität-GH Siegen, Adolf-Reichwein-Str., D-57068 Siegen, Germany. E-mail: [email protected]
Received: 21 February 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
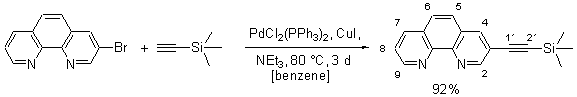
The experimental procedure follows a protocol developed by Sonogashira [1] and recently used for the preparation of macrocyclic phenanthrolines.[2] All reactions were carried out under an atmosphere of dry argon by using standard Schlenk tube techniques. At first a solution of 3-bromo-[1,10]phenanthroline [3] (4.60 g, 18.0 mmol) and trimethylsilanylethyne (5.30 g, 54.0 mmol) in benzene (30 mL) and dry triethylamine (15 mL) was prepared. After addition of PdCl2(PPh3)2 (630 mg, 900 µmol) and CuI (523 mg, 1.79 mmol) the resulting mixture was kept at reflux for three days. After removal of the solvent, the black residue was dissolved in dichloromethane (150 mL), washed with aqueous potassium cyanide (2%, 100 mL) and with water (100 mL). The organic layer was dried over MgSO4 and then purified by column chromatography (SiO2, 1. CH2Cl2, 2. diethylether, Rf (Et2O) = 0.10) to furnish 4.57 g (92%) of the title compound as colorless crystals.
Mp. 136 °C.
IR (KBr): n= 3056, 2956, 2154 (Cº C), 1589, 1494, 1420, 1248, 1100, 863, 838, 731, 656 cm-1.
1H NMR (CDCl3, 250 MHz): d = 0.29 (s, 9 H, Si(CH3)3), 7.58 (dd, J = 8.0 Hz, J = 4.3 Hz, 1 H, 8-H), 7.63 (d, J = 9.8 Hz, 1 H, 6-H), 7.74 (d, J = 9.8 Hz, 1 H, 5-H), 8.18 (dd, J = 8.0 Hz, J = 1.8 Hz, 1 H, 7-H), 8.28 (d, J = 2.1 Hz, 1 H, 4-H), 9.15 (dd, J = 4.3 Hz, J = 1.8 Hz, 1 H, 9-H), 9.16 (d, J = 2.1 Hz, 1 H, 2-H).
13C NMR (CDCl3, 53 MHz): d = -0.2 (Si(CH3)3), 99.4 (C-1'), 101.7 (C-2'), 119.4 (C-3), 123.2 (C-8), 125.9 (C-4a), 127.2 (C-6a), 127.5 (C-5), 128.9 (C-6), 136.0 (C-7), 138.6 (C-4), 144.7 (C-10a), 145.8 (C-1a), 150.5 (C-9), 152.3 (C-2).
Anal. Calcd for C17H16N2Si · 0.25 H2O, C: 72.69, H: 5.92, N: 9.97. Found C: 72.63, H: 6.04, N: 10.06.
Acknowledgements: The authors gratefully acknowledge financial support from the DFG, the Volkswagenstiftung and the Fonds der Chemischen Industrie.
References
1. Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron
Lett. 1975, 4467.
2. Schmittel, M.; Ammon, H. Synlett. 1999,
750.
3. Tzalis, D.; Tor, Y.; Failla, S.; Siegel, J.S.
Tetrahedron
Lett. 1995, 36, 3489.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/