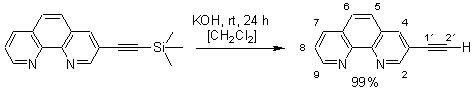
3-Ethynyl-[1,10]phenanthroline
Christoph Michel, Davood Habibi and Michael Schmittel*
FB 8 - OC 1 (Chemie - Biologie), Universität GH Siegen, Adolf-Reichwein-Str., D-57068 Siegen, Germany, E-mail: [email protected]
Received: 21 February 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
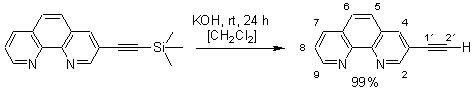
The experimental procedure follows a protocol developed by Eaborn [1]. To 3-trimethylsilanylethinyl-[1,10]phenanthroline (500 mg, 21.7 mmol), dissolved in THF (10 mL), was added 1 M KOH in methanol (10 mL). The resultant solution was stirred for 24 hours at room temperature. After addition of water (10 mL) the product was extracted with CH2Cl2 (3 x 10 mL). Removal of the solvent in vacuo afforded 426 mg of 3-ethynyl-[1,10]phenanthroline (96%) as a colorless solid.
Mp. 279 °C.
IR (KBr): ![]() = 3140 (s,
C-H), 2086 (w, Cº C), 1588 (m, C=C), 1551
(m, C=C), 1499 (s, C=C), 1418 (s), 1264 (m), 1222 (s), 1096 (m), 904 (m),
838 (s, Ar-H), 729 (s, Ar-H) cm-1.
= 3140 (s,
C-H), 2086 (w, Cº C), 1588 (m, C=C), 1551
(m, C=C), 1499 (s, C=C), 1418 (s), 1264 (m), 1222 (s), 1096 (m), 904 (m),
838 (s, Ar-H), 729 (s, Ar-H) cm-1.
1H NMR (CDCl3, 250 MHz): d = 3.34 (s, 1 H, 2'-H), 7.58 (dd, J1 = 8.3 Hz, J2 = 4.1 Hz, 1 H, 8-H), 7.66 (d, J = 8.8 Hz, 1 H, 5-H), 7.73 (d, J = 8.8 Hz, 1 H, 6-H), 8.15 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1 H, 7-H), 8.27 (d, J = 2.2 Hz, 1 H, 4-H), 9.14 (dd, J1 = 4.1 Hz, J2 = 1.5 Hz, 1 H, 9-H), 9.17 (d, J = 2.2 Hz, 1 H, 2-H).
13C NMR (CDCl3, 53 MHz): d = 80.5 (C-1'), 81.5 (C-2'), 118.3 (C-3), 123.2 (C-8), 125.8 (C-4a), 127.3 (C-6a), 127.4 (C-5), 128.9 (C-6), 136.0 (C-7), 138.9 (C-4), 144.9 (C-10a), 145.6 (C-1a), 150.4 (C-9), 152.2 (C-2).
Anal. Calcd for C14H8N2, C: 82.33, H: 3.95, N: 13.72. Found C: 81.89, H: 3.92, N: 13.85.
Acknowledgements: The authors gratefully acknowledge financial support from the DFG, the Volkswagenstiftung and the Fonds der Chemischen Industrie.
Reference
1. Eaborn,C.; Walton, D. R. M. J. Organometal. Chem. 1965, 4, 217.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/