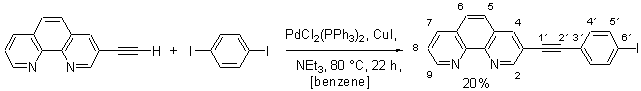
3-(4-Iodo-phenylethynyl)-[1,10]phenanthroline
Christoph Michel, Davood Habibi and Michael Schmittel*
FB 8 - OC 1 (Chemie-Biologie), Universität GH Siegen, Adolf-Reichwein-Str., D-57068 Siegen, Germany. E-mail: [email protected]
Received:21 February 2001 / Accepted: 15 May 2001 / Published: 25 May 2001
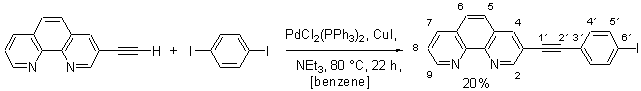
The experimental procedure follows a protocol developed by Sonogashira [1]. All reactions were carried out under an atmosphere of dry argon by using standard Schlenk tube techniques. To a mixture of 3-ethynyl-[1,10]phenanthroline (309 mg, 1.50 mmol), 1,4-diiodobenzene (2.47 g, 7.50 mmol), CuI (225 mg, 1.77 mmol) and [PdCl2(PPh3)2] (75.0 mg, 99.7 m mol) in dry benzene (25 mL) and dry NEt3 (10 mL). The reaction mixture was kept at 80 °C for 22 h while stirring vigorously. After removal of the solvent the residue was washed with aqueous KCN (2%, 30 mL) and purified by column chromatography (SiO2, CHCl3, Rf = 0.21) to furnish 3-(4-iodo-phenylethynyl)-[1,10]phenanthroline as a yellow solid (122 mg, 20%).
Mp. > 300 °C.
IR (KBr): ![]() = 3205 (s,
C-H), 2202 (w, Cº C), 1590 (m, C=C), 1477
(s, C=C), 1415 (s), 1261 (m), 1095 (m), 1053 (m), 1202 (s), 940 (m), 818
(s, Ar-H), 729 (s, Ar-H) cm-1.
= 3205 (s,
C-H), 2202 (w, Cº C), 1590 (m, C=C), 1477
(s, C=C), 1415 (s), 1261 (m), 1095 (m), 1053 (m), 1202 (s), 940 (m), 818
(s, Ar-H), 729 (s, Ar-H) cm-1.
1H NMR (d6-acetone, 250 MHz): d = 7.47 (d, J = 8.6 Hz, 2 H, 4'-H), 7.77 (dd, J1= 8.3 Hz, J2 = 4.0 Hz, 1 H, 8-H), 7.88 (d, J = 8.6 Hz, 2 H, 5'-H), 7.98 (d, J = 8.9 Hz, 1 H, 5-H), 8.04 (d, J = 8.9 Hz, 1 H, 6-H), 8.47 (dd, J1 = 8.3 Hz, J2 = 1.5 Hz, 1 H, 7-H), 8.62 (d, J = 2.2 Hz, 1 H, 4-H), 9.14 (dd, J1 = 4.0 Hz, J2 = 1.5 Hz, 1 H, 9-H), 9.19 (d, J = 2.2 Hz, 1 H, 2-H).
13C NMR (d6-acetone, 53 MHz): d = 88.6 (C-2'), 93.0 (C-1'), 95.5 (C-6'), 119.7 (C-3), 123.0 (C-8), 124.3 (C-4a), 127.1 (C-6a), 128.6 (C-5), 128.8 (C-6), 130.2 (C-4'), 134.2 (C-5'), 136.9 (C-7), 138.9 (C-3'), 139.0 (C-4), 146.0 (C-10a), 146.9 (C-1a), 159.4 (C-9), 160.5 (C-2).
Anal. Calcd for C20H11IN2, C: 59.13, H: 2.73, N: 6.90. Found C: 58.84, H: 2.56, N: 6.86.
Acknowledgements: The authors gratefully acknowledge financial support from the DFG, the Volkswagenstiftung and the Fonds der Chemischen Industrie.
Reference
1. Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 4467.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/