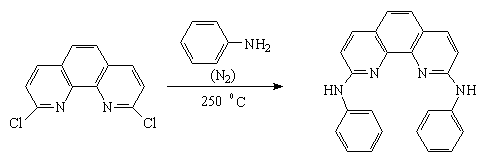
2, 9-Diphenylamino-1,10-phenanthroline
Hai-Yang Liu, Chi-Fong Luo, Kuo-Shen Chuang and Wen-Jwu Wang*
Department of Chemistry, Tamkang University, Taipei 25137, Taiwan.Tel. +886-02-26215656-2530, E-mail: [email protected]
Received: 5 February 2001 / Accepted: 15 May 2001 / Published: 25 May 2001

2,9-Dichloro-1,10-phenanthroline was prepared by a previously published method [1]. 2,9-Dichloro-1,10-phenanthroline (50 mg) was placed in a flask where the temperature was raised 150 °C with oil-bath, and aniline was introduced under N2 gas. The temperature was raised to 250 °C and maintained for 5 hours. The mixture was purified by recrystalization from methanol, to give 2,9-diphenylamino-1,10-phenanthroline as a dark brown crystal (30 mg, yield: 30.0%).
1H-NMR (300MHz, d-TFA/CDCl3, V:V=1:1): 8.73 (2H, d, J=9.5), 8.24 (2H, s), 7.87 (6H, t), 7.69 (6H, t).
IR (KBr, cm-1): 410 (w), 500 (w), 589 (w), 613 (w), 689 (w), 730 (w), 762 (m), 846 (m), 1028 (w), 1076 (w), 1153 (w), 1209 (w), 1257 (w), 1284 (w), 1327 (w), 1362 (m), 1386 (w), 1397 (w), 1441 (m), 1453 (s), 1491 (m), 1510 (m), 1547 (s), 1577 (m), 1599 (m), 1642 (s), 1649 (s), 3279 (w), 3321 (m).
UV-Vis (l , nm, in CH3OH): 206.5, 225.5, 277.5, 347.5.
FAB-MS ([M+1]+): 363.
Acknowledgement: Support for this research provided by the National Science Council, Taiwan is gratefully acknowledged.
Reference
1. Ogawa, S.; Yamaguchi, T.; Gotoh, N. J. Chem. Soc. Perkin Trans. 1974, 976-978.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/