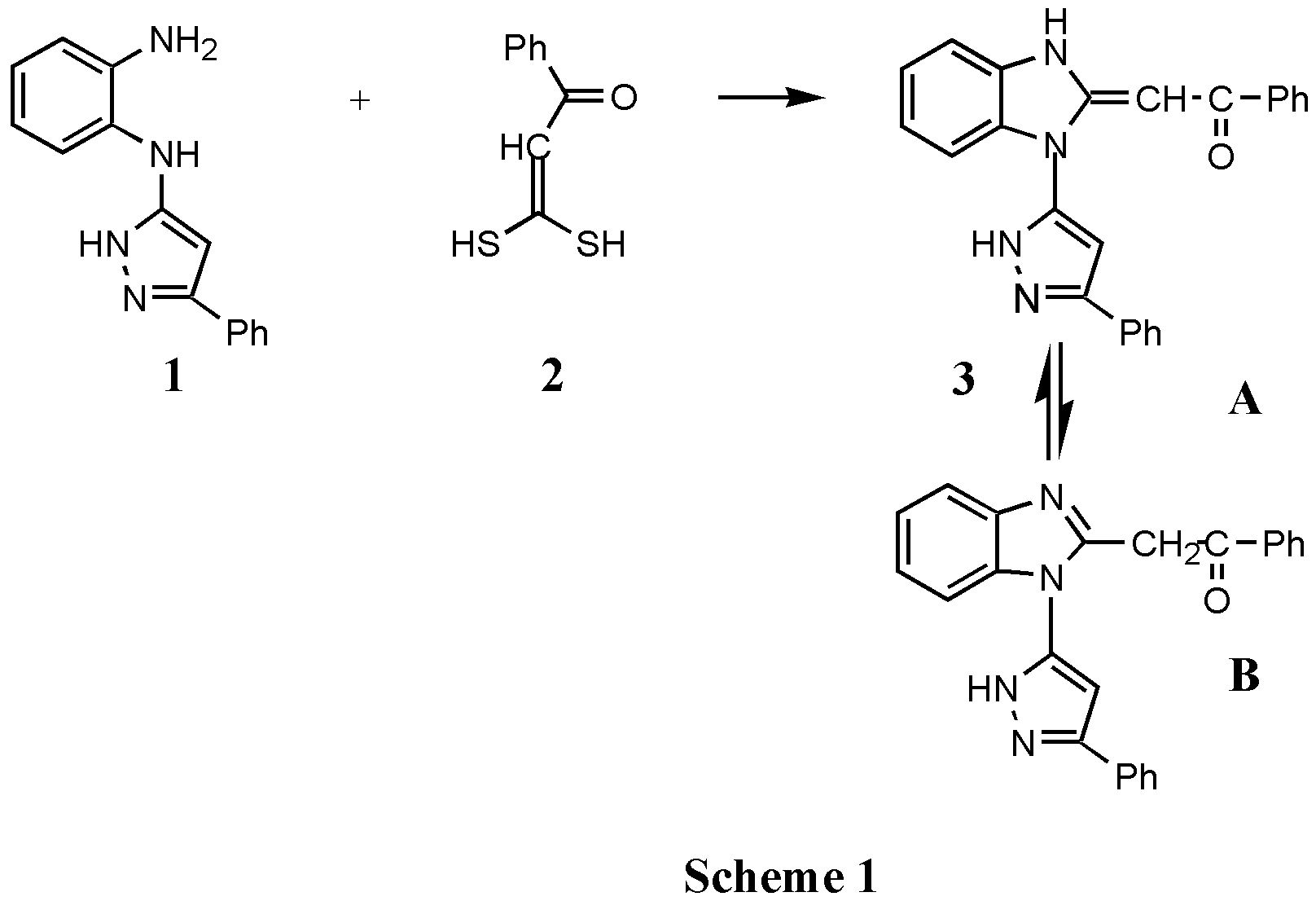
(3(5) Phenylpyrazol-5(3)-yl)-2-benzoylmethyl Benzimidazole
Noureddine Hammou Ahabchane, Joseph Téné Ghomsi and El Mokhtar Essassi*
Laboratoire de chimie organique hétérocyclique, Faculté des sciences, Avenue Ibn- Batouta, Rabat, Maroc. E-mail: [email protected]
Received: 12 January 2001 / Accepted: 15 May 2001 / Published: 25 May 2001

The compound 3, which exists in tautomeric equilibria between enamine (form A) and methylene imine (form B) as schown in scheme 1, was prepared by addition of the 3,3-dimercapto-1-phenyl-prop-2-ene-1-one 2 to 3-N-(2-aminophenylamino)-5-phenylpyrazole 1 [1]. A mixture of dimercapto propenone 2 (1.96 g, 0.01 mol) and 3-N (2-aminophenylamino)-5-phenylpyrazole 1 (2.50 g , 0,01mol) in xylene (60 mL) was refluxed for 2 h. After removal of the solvent, the residue was purified by crystallization (ethanol) to afford 3 in good yield (3.02 g, 80%).
Mp.: 166-168 °C.
MS DCI (NH3): [M+H] +: 379.
IR (KBr, cm-1): 1680(vC=0), 3372 (nNH).
1H NMR (DMSOd6) d : 13.79 (s, 1H, NH), 13.50(s, 1H, NH), 9.14 (s, 1H, NH), 7.99-7.01 (m, 14H), 6.80 (s, 1H, C4íH), 6.19 (s, 1H, C2=CH), 3.40 (s, 2H, C2-CH2).
Anal. Calc. For C24H18N4O (378): C 76.19, H 4.76, N 14.81; Found: C 76.05, H 4.73, N 14.32.
Reference
1. Essassi, E. M.; Salem, M.; Bull. Soc. Chim. Belg. 1985, 94, 755.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/