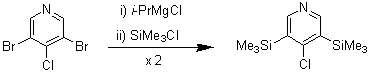
3,5-Bis(trimethylsilyl)-4-chloropyridine
A.J. Kay and A.D. Woolhouse*
Industrial Research Ltd., P.O.Box 31 310, Lower Hutt, New Zealand
E-mail:
[email protected]
Received: 8 June 2001 // Accepted: 14 December 2001 / Published 20 December 2001
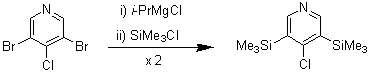
In connection with our studies of the nonlinear optical properties of pyridinylidene compounds we required some pyridine derivatives bearing bulky substituents at the 3- and 5-positions in addition to having an efficient leaving group at the 4-position. To this end we synthesised 3,5-bis(trimethylsilyl)-4-chloropyridine using a bromine-magnesium exchange method recently described [1].
To a stirred solution of 3,5-dibromo-4-chloropyridine [2] (5.27 g, 19.4 mmol) in anhydrous thf (20 mL) under a N2 atmosphere and at room temperature was added dropwise a solution of isopropyl magnesium chloride in thf (10.2 mL, 1.91 M, 19.5 mmol). After 1 h, chlorotrimethylsilane (2.12 g, 2.47 mL, 19.5 mmol) was added and the mixture stirred overnight at room temperature. A further portion of isopropyl magnesium chloride (19.5 mmol) was then added, followed by a second addition of chlorotrimethylsilane (19.5 mmol) 1 h later. The mixture was stirred overnight, poured into water (50 mL) and extracted with dichloromethane (3 x 50 mL). The combined organic extracts were washed with water (50 mL), dried (MgSO4) and concentrated under vacuum to a brown oil. This was subjected to flash chromatography (hexanes elution) to give 3,5-bis(trimethylsilyl)-4-chloropyridine as a white solid (3.47 g, 70 %). Recrystallisation (benzene) gave colourless crystals.
M.p. 68-70 ° C.
MS: Found: MH+ m/z 258.08984 C11H20ClNSi2 requires 258.08956 D = 0.8 p.p.m.
1H NMR (300 MHz, CDCl3): d 0.20, s, 18 H; 8.33, s, 2H.
13C NMR (75 MHz, CDCl3): d 0.0 (CH3), 134.5 (CQ), 156.9 (CH), 158.9 (CQ).
IR (nujol): 1535, 1404, 1250, 1055.
References
1. Trecourt F.; Breton G.; Bonnet V.; Mongin F.; Marsais F.; Queguiner
G. Tetrahedron 2000, 56, 1349.
2. den Hertog, H. J.; Hoogzand, C. Recl. Trav. Chim Pays-Bas1957,
76,
261.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/