4-(Benzylsulfonyl)acetophenone
Bruce A. Hathaway* and Mary M. Triefenbach
Department of Chemistry, Southeast Missouri State University, MS 6400, One University Plaza, Cape Girardeau, Missouri, 63701, USA. Tel. 001 573-651-2370; Fax 001 573-986-6433. E-mail: [email protected]
Received: 28 May 2001 / Accepted: 14 December 2001 / Published: 20 December 2001
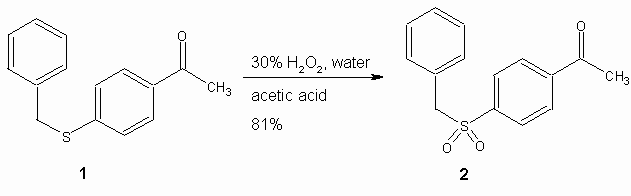
During the course of our research on preparing parallel dipole-aligned crystals [1-2], we needed to prepare the previously unreported compound 4-benzyl-sulfonylacetophenone (2). For this purpose we developed a synthesis via the compound 1 [3], prepared in turn from commercially available 4-fluoro-acetophenone. Oxidation of 1 with 30% H2O2 in acetic acid/water readily formed the title compound, 2. The overall yield for the two steps was 76%. Starting material and product were easily distinguished by 13C-NMR spectroscopy: while the positions of the benzylic CH2ís of 1 and 2 were quite similar in the 1H-NMR spectra (4.20 and 4.35 ppm, respectively), the benzylic carbons were very different in the 13C-NMR spectra (37.52 and 63.19 ppm, respectively).
Following the general procedure in Organicum [4], 0.50 g (2.1mmol) of 4-(benzylthio)acetophenone [3] was dissolved in 20 mL of acetic acid. To this solution was added 2.0 mL of 30% aqueous hydrogen peroxide dropwise while stirring. The reaction was monitored by TLC (silica gel, ethyl acetate). After 30 minutes, TLC indicated starting material was still present (Rf = .66), and two other spots were present (Rf = .44 and .55), presumably the sulfone and the sulfoxide. After one hour, only the Rf = .44 spot was present. After standing overnight, a white precipitate had formed. The reaction mixture was poured into 20 mL of ice water, then the solid was filtered and washed with water.
Yield: 0.46 g (81%).
Melting point: 170-172 °C.
IR (KBr pellet, cm-1): 3056, 2993, 2943, 1683, 1316, 1291, 1151, 1089, 842, 775, 697, 653, 527.
1H-NMR (300 MHz, CDCl3, ppm): 7.99 (2H, doublet, J=8.3 Hz), 7.72 (2H, doublet, J=8.3 Hz), 7.29 (3H, multiplet), 7.09 (2H, doublet, J=7.0 Hz), 4.35 (2H, singlet), 2.64 (3H, singlet).
13C-NMR (75.5 MHz, CDCl3, ppm): 197.21, 141.98, 141.17, 131.19, 129.49, 129.42, 129.14, 128.97, 128.01, 63.19, 27.33.
GC-MS (m/e, relative intensity): 91 (100%), 65 (19%), 275 (trace, MH+); all other peaks were <10%.
Acknowledgement: Acknowledgment is made to the donors of the Petroleum Research Fund, administered by the American Chemical Society, for partial support of this research.
References
| 1. | Hathaway, B. A.; Day, G.; Lewis, M.; Glaser, R. Synthesis, Structure, Electrostatic Properties and Spectroscopy of 3-Methyl-4,5,6,7-tetrafluoro-1H-indazole. An Experimental and ab initio Computational Study. J. Chem. Soc., Perkin Trans. 2, 1998, 2713-2719. |
| 2. | Lewis, M.; Barnes, C. L.; Hathaway, B. A.; Glaser, R. 4-Methoxybenzylidene pentafluoro-phenylmethylidenehydrazone. Acta Cryst. 1999, C55, 975-978. |
| 3. | Hathaway, B.A.; Triefenbach, M.M. Molecules 2001, 6, M244. |
| 4. | Ongley, P.S., Ed. Organicum: Practical Handbook of Organic Chemistry. Addison-Wesley Publishing Company, Inc, Reading, MA. 1973; p 637. |
Sample availability: available from MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/