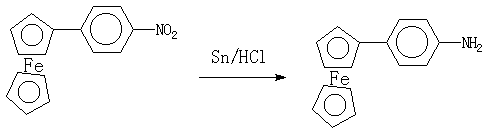
4-Ferrocenylaniline
Hu Ping, Ke-Qing Zhao* and Hong-Bo Xu
Department of Chemistry, Sichuan Normal University, Chengdu, 610066,
China
Tel./Fax: 86-28-4764743. Email: [email protected] (Present e-mail: [email protected])
Received: 28 May 2001 / Accepted: 14 December 2001 /Published: 20 December 2001
Keywords: 4-nitrophenylferrocene, 4-ferrocenylaniline, reduction, metallomesogen, non-linear optical material, intermediate, tin
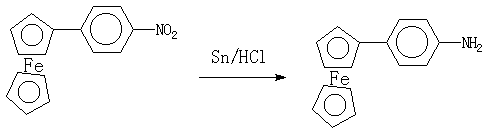
The reduction of 4-nitropheneylferrocene with tin in acidic condition gives 4-ferrocenylaniline, which is an important intermediate for the synthesis of ferrocene-containing Schiff's base liquid crystals [1].
To a stirred mixture of 4-nitrophenylferrocene [2] (2 g, 7 mmol) in 25 ml of concentrated hydrochloric acid and 40 ml ethanol is added tin (granulated, 4.5 g, 40 mmol) and the reaction mixture is heated under reflux for 4 h. After the mixture has cooled, 200 ml water is added and aqueous NaOH is added to adjust the pH to 14 before filtration. The filtrate is extracted with CH2Cl2 and dried (Na2SO4). The solvent is removed by rotary evaporation. The crude product is recrystallized from petroleum ether (boiling range 60-80oC) to give 4-ferrocenylaniline as an red-orange solid (1.4 g, 76%).
M.p: 157-159oC.
IR(KBr, cm-1): 3437, 3350, 1621, 1605, 1529, 1454, 1103, 998.
1HNMR(CDCl3): 7.3(d, 2H, C6H4), 7.65(d, 2H, C6H4), 4.0(s, 5H, C5H5), 4.2(s, 2H, C5H4),
4.5(s, 2H, C5H4), 3.4(s, 2H, NH2).
Elemental analysis for C16H15FeN: calculated, C, 68.57; H, 5.38; N, 5.00%. Found: C, 68.85; H, 5.46; N, 5.12%.
References
1. Espinet, P.; Esteruelas, M. A.; Oro, L. A.; Serrono,
J. L.; Sola, E. Coor. Chem. Rev. 1992, 117, 215.
2. Hu, P.; Zhao, K.-Q.; Xu, H.B. Molecules 2001,
6,
M249.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/