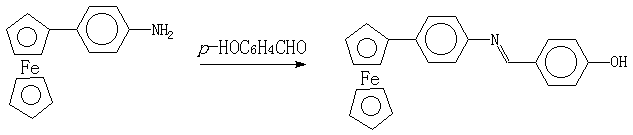
(4-hydroxybenzylidene)-4-ferrocenylaniline
Ping Hu, Ke-Qing Zhao* and Hong-Bo Xu
Department of Chemistry, Sichuan Normal University, Chengdu, 610066,
China
Tel./Fax: 86-28-4764743. Email: [email protected] (Present e-mail: [email protected])
Received: 28 May 2001 / Accepted: 14 December 2001 /Published: 20 December 2001
Keywords: liquid crystal, non-linear optical material, metallomesogen, ferrocene, 4-ferrocenylaniline, 4-hydroxybenzaldehyde, condensation reaction, (4-hydroxybenzylidene)-4-ferrocenylaniline, Schiff 's base
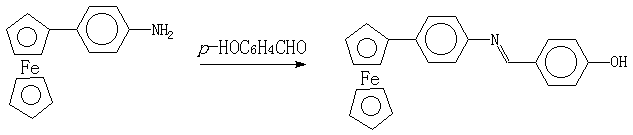
(4-Hydroxybenzylidene)-4-ferrocenylaniline has a free hydroxyl group and it can react with carboxylic acids to form esters and it is thus a key intermediate for the synthesis of mono-substituted ferrocene-containing liquid crystal [1] with Schiff's base and ester group. It can be synthesized by the condensation reaction of 4-ferrocenylaniline with 4-hydroxybenzaldehyde.
A stirred mixture of 4-ferrocenylaniline [2] (8.31g, 30mmol) and 4-hydroxybenzaldehyde(3.73 g, 30mmol) in 50 ml ethanol is heated under reflux for 2 h. The mixture is cooled and the precipitated product is collected by filtration as a yellow powder (10.5 g, 91%). The product is pure enough for analysis and for further use without further purification.
M.p: ca. 270°C (blackens and decomposes before melting).
IR(KBr, cm-1): 3465, 3070, 1645, 1584, 1530.
1HNMR (DMSO-d6, 300Hz): 10.11(1H, s, OH), 8.53(1H, S, CH=N), 4.02(5H, s, C5H5), 4.35-4.79(4H, d, C5H4), 6.88-7.80(4H, dd, J=9.0Hz, C6H4-OH), 7.16-7.56(4H, dd, J=8.9Hz, Fc-C6H4).
Elemental analysis for C23H19FeNO, calculated: C, 71.88; H, 4.95; N, 3.65%.
Found: C, 71.23; H, 4.84; N, 3.55%.
References
1. Espinet, P.; Esteruelas, M. A.; Oro, L. A.; Serrono,
J. L.; Sola, E. Coor. Chem. Rev. 1992, 117, 215.
2. Hu, P.; Zhao, K.-Q.; Xu, H.B. Molecules 2001,
6,
M250.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/