Reduction of 5-Nitrosalicylic Acid in Water to Give 5-Amino-salicylic Acid*
Gabriele Breviglieri 1* Bruno Giacomo 1, Contrini Sergio 1, Assanelli Cinzia 1, Eileen Campanab2 and Mauro Panunzio 2*
1. Farchemia s.r.l , Via Bergamo 121 I-24047 Treviglio (Bg), Italy
2. I.Co.E.A.-CNR, Via Gobetti 101, 40121 Bologna, Italy. Email: [email protected]
Received: 28 May 2001 / Accepted: 15 December 2001 / Published: 20 December 2001

In the course of a general programme aimed to find new protocols for environmentally friend chemistry [1-3] we were looking for a clean, high yielding method of reduction of 5-nitrosalicylic acid (1) to 5-aminosalicylic acid (5-ASA) in industrial scale [4].This product covers a large range of industrial applications: use as a starting material for the synthesis of biologically active compounds for pharmaceutical industries (eg Asacol) or 5-N-pyrrylsalicylic acid [5], to intermediate for dyes [6], or polymeric compounds which have potential applications in ion-exchange chromatography of cationic materials and selective binding of positively charged molecules [7]. For the pharmaceutical industry alone the needs are up to 300 tons/year. A literature survey, including patent literature, revealed several methods, for its preparation, all of which include the use of poisonious organic solvents and/or reagents difficult to be removed from the reaction mixture.
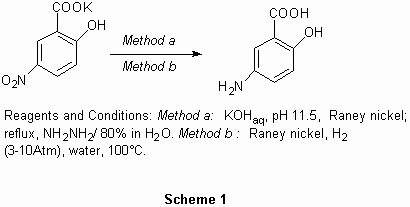
We have found that high yielding reduction may be performed in water following the two methods reported below (Scheme 1). The methods present the advantage of using the environmentally friendly solvent, water, ease of work-up, no need for a time-consuming and expensive purification procedure, and is applicable on an industrial scale. Moreover Method B avoids the use of hydrazine hydrate, since the reaction can be performed in autoclave under 3-10 atmospheres of hydrogen pressure. The yield and quality of the final product are the same as those obtained using hydrazine hydrate.
In conclusion, the methods herein described constitute, in our opinion, an improvement over the existing methods and represent a contribution to highly desirable, environmentally friendly chemistry.
Procedure A: To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. To this solution 2 g of Raney nickel (Degussa B113W) were added. The mixture was heated-up to reflux and hydrazine hydrate (40 mL, 80% in water, 64 mmol) was added dropwise during 3-4 hrs. The reflux was maintened until HPLC showed the disappearance of the starting material and the complete reduction of 1 (3-4 hrs). The hot mixture was filtered under nitrogen and the solution was collected. The solution was cooled to 40°C and the pH was adjusted to 2.3 by addition of 35% HCl aqueous solution (w/v). The precipitation of 5-ASA occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with water, and dried at 60-70°C. 5-ASA was obtained in 89% yield (34 g, 220 mmol).This product was conformed to the USP specifications.
Procedure B
To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. The solution was charged in a stainless steel autoclave and 2 g of Raney nickel (Degussa B113W) are added. Hydrogen was introduced into the autoclave reaching a pressure of 8 atm.The mixture was heated-up to 100°C. The temperature was maintained until HPLC-test showed the disappearance of the starting material and the complete reduction of (1) (6 -8 hrs). Hydrogen was purged and replaced by nitrogen. The hot mixture was filtered under nitrogen, the filtrate was cooled to 40°C, and the pH was adjusted to 2.3 by addition of 35% (w/v) HCl aqueous solution. The precipitation of the 5-ASA occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with ion depleted water, and dried at 60-70°C. 5-ASA was obtained in 89% yield (34 g, 220 mmol).
This procedure was successfully applied in industrial scale using a 6000 litre autoclave and reducing 800 kg of 1 per batch.
References
1. Panunzio, M.; Castiglioni, E.;
Campana, E.; Favi, G.; Vicennati, P. Application of Microwaves Technique
to Organic Synthesis; Acierno, D., Leonelli, C. and Pellacani, G. C.,
Ed.; Mucchi Editore: Modena, 2000.
2. Gianotti, M.; Martelli, G.; Mendozza,
M. Synthetic Communications 2000, 30, 1725.
3. Martelli, G.; Spunta, G.; Panunzio,
M. Tetrahedron Lett. 1998, 39, 6257-6260.
4. For lab-scale synthesis see:
a) Meshram, H. M.; Ganesh, Y. S. S.; Sekhar, K. C.; Yadav, J. S. Syn.
Lett. 2000, 7, 993-994. b)Stutts, K. J.; Scortichini,
C. L.; Repucci, C. M. J. Org. Chem. 1989, 54, 3740-3744.
and Ref. therein cited.
5. CA 81/151984x, CA 81/63478
6. See for example: CA 87/137320v,
CA 85/192730n
7. Kennedy, J.F.; Barker, S.A.;
Epton, J.; Kennedy, G.R.; J. Chem. Soc. Perkin 1, 1973, 488-490.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/