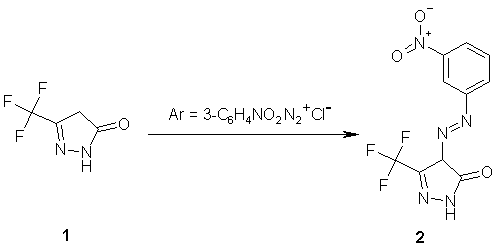
4-(3-Nitrophenylazo)-5-trifluoromethyl-2,4-dihydropyrazol-3-one
Hussein F. Zohdi1*, Nora M. Rateb2 and A. Z. Haikal1
1.Department of Chemistry, Faculty of Science, United Arab Emirates
University, P.O.Box 17551 Al-Ain, UAE
E-mail: [email protected]
2.Department of Chemistry, Faculty of Science, Cairo University, Giza,
Egypt
Received: 5 June 2001 / Accepted: 15 December 2001 / Published: 20 December 2001
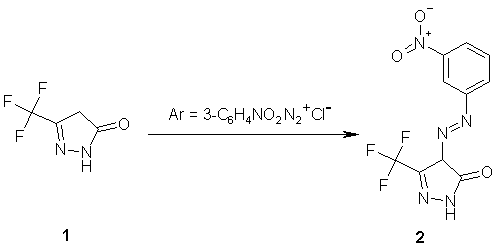
To a cold solution of 5-trifluoromethyl-2,4-dihydropyrazol-3-one [1,2] (0.76 g, 5 mmol) in ethanol (25 ml) containing sodium acetate (0.82 g, 10 mol), 3-nitrobenzenediazonium chloride ( 5 mmol) was added dropwise with stirring at 0-5°C. The reaction mixture was stirred at room temperature for three hours and the precipitated crude product was filtered, washed with water, dried and crystallized from ethanol to give 1.2 g (80%) of 2 as orange crystals.
M.p. 253-255oC.
MS (m/z): 301.
1H-NMR (250 MHz, DMSO-d6): 2.50(s, 1H, CH); 7.71-8.45(m, 4H, aromatic CH); 12.70(s, 1H, NH).
13C-NMR (75 MHz, CDCl3): 116.65, 124.24(quaternary aromatic carbons); 111.04, 119.99, 122.40, 130.77(4 aromatic CH’s); 136.50(q, CF3); 142.38(C-4); 148.35(C-5); 158.33(CO).
References
1. Poulter, C. D.; Wiggins, P. L.; Plummer, T. L. J. Org. Chem. 1981,
46,
1532.
2. Zohdi, H. F.; Elghandour, A. H. H.; Rateb, N. M.; Sallam, M.M.M.
J.
Chem. Res. 1992, (S) 396; (M) 3015.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/