Molecules
2001,
6,
M262
2-(2`,3`,5`-Tri-O-acetyl-b
-D-ribofuranosyl)-4-(3-nitrophenylazo)-5-trifluoromethyl-2,4-dihydropyrazol-3-one
Abdelfattah Haikal, Hussein F. Zohdi* and Shaikha El-Neyadi
Department of Chemistry, Faculty of Science, United Arab Emirates University,
P.O.Box 17551 Al-Ain, UAE
E-mail: [email protected]
and
[email protected]
Received: 5 June 2001 / Accepted: 15
December 2001 / Published: 20 December 2001
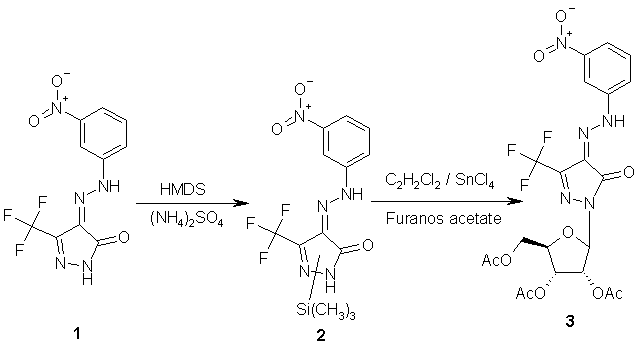
To a solution of 4-(3-nitrophenylazo)-5-trifluoromethyl-2,4-dihydropyrazol-3-one
1
[1] (0.903 g, 3 mmol) in hexamethyldisilazine (HMDS) (25 ml) was added
few crystals of anhydrous ammonium sulfate [2]. The mixture was refluxed
for three hours, then it was evaporated under vacuum to dryness. The residue
was mixed with anhydrous xylene (30 ml) and the resulted solution was re-evaporated
under vacuum to dryness to remove the remaining traces of HMDS. To a solution
of the residue in anhydrous 1,2-dichloromethane (25 ml) was added 1,2,3,5-tetra-O-acetyl-b-D-ribofuranose
(0.954g, 3 mmol). The mixture was treated with SnCl4 (1.1 mmol)
[2] and was then stirred at room temperature for two hours (tlc). The reaction
mixture was diluted with dichloromethane (25 ml), washed with saturated
aqueous solution of sodium bicarbonate (50 ml) and water (3x30 ml). The
organic layer was dried over anhydrous sodium sulfate, filtered, evaporated
to a small volume and chromatographed over silica gel column using ethyl
acetate / n-hexane (4:6 v/v) to give 1.34g (80%) of 3 as yellow
powder.
Rf (ethyl acetate/n-hexane, 50/50, v/v): 0.3.
UV (lmax , 95% ethanol): 266,
404.
MS (m/z): 559.
1H-NMR (250 MHz, CDCl3): 2.07(s, 9H, COCH3);
4.07-4.13(dd, 1H, H-5``); 4.32-4.27(m, 1H, H-4`); 4.36-4.41(dd, 1H, H-5`,
J5`,4`=3.3, J5`,5``=12.27); 5.44-5.48(t, 1H, H-3`,
J3`,4`=5.31); 5.62-5.65(dd, 1H, H-2`, J2`,3`=5.67);
5.95(d, 1H, H-1`, J1`,2`=3.84); 7.56-8.22(m, 4H, aromatic CH).
13C-NMR (75 MHz, CDCl3): 70.65(C-5`), 72.48(C-3`);
76.99(C-2`); 79.75(C-4`); 84.44(C-1`); 111.52, 117.81, 120.36, 122.85,
124.50, 131.11(6 aromatic carbons); 136.60(q, CF3); 142.74(C-4);
148.56(C-5); 157.68(C-3); 169.40, 169.60, 170.63(3 CO).
References
1. Zohdi, H.F.; Rateb, N.M.; Haikal, A. Molecules 2001,
6, M261.
2. Vorbruggen, H.; Bennua, B. Chem. Ber. 1981, 114,
1279.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
