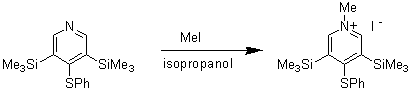
3,5-Bis(trimethylsilyl)-1-methyl-4-thiophenoxypyridinium Iodide
A.J. Kay and A.D. Woolhouse*
Industrial Research Ltd., P.O.Box 31 310, Lower Hutt, New Zealand. E-mail: [email protected]
Received: 8 June 2001 / Accepted: 15 December 2001 / Published: 20 December 2001
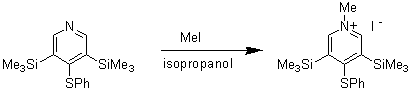
3,5-Bis(trimethylsilyl)-4-thiophenoxypyridine is readily converted to its pyridinium methiodide. The product is a precursor to "stunted" pyridylidene merocyanines that have bulky substituents at the 3- and 5-positions. Incorporation of such bulk is expected to afford chromophores with exceptionally high non-linear optical responses as a result of twisting between the donor and acceptor moieties [1].
A solution of 3,5-bis(trimethylsilyl)-4-thiophenoxypyridine [2] (1.0 g, 3.02 mmol) and methyl iodide (2.5 g, 17.6 mmol) in isopropanol (10 mL) was refluxed with stirring overnight, cooled and concentrated under vacuum to half its original volume. The resulting orange-yellow precipitate was recovered by filtration and washed with a little isopropanol to give a bright yellow solid (1.18 g, 83%) that was suitable for use without further purification. An analytical sample was prepared by recrystallisation from isopropanol to give the desired pyridinium iodide as bright yellow hairs.
M.p. 259-261 °C.
1H NMR (300 MHz, d6-DMSO): 0.27, s, 18H; 4.42, s, 3H; 6.92-6.94, m, 2H; 7.20-7.25, m, 1H; 7.29-7.34, m, 2H.
13C NMR (75 MHz, d6-DMSO): d 0.0 (CH3), 48.2 (CH3), 127.1 (CH), 127.2 (CH), 130.1 (CH), 137.5 (CQ), 147.0 (CQ), 151.0 (CH), 163.6 (CQ).
Anal. Calc for C18H28INSSi2 C 45.65, H 5.96, N 2.96. Found C 45.45, H 5.89, N 2.90.
IR (nujol): 1603, 1578, 1521, 1476, 1372, 1246, 924, 846, 774.
References
1. Albert, I. D. L.; Marks, T. J.; Ratner, M. A. J. Am. Chem. Soc.
1998, 120, 11174
2. Kay, A. J.; Woolhouse, A. D. Molecules 2001, 6, M266.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/