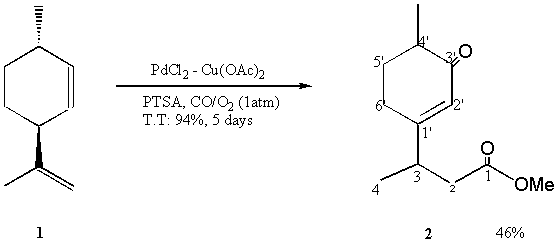
3-(4’-Methyl-3’-oxocyclohex-1’-enyl)butyric Acid Methyl Ester
Soufiane El Houssame*, Larbi El Firdoussi and Abdellah Karim
Laboratoire de Chimie de Coordination, Faculté des Sciences Semlalia, BP 2390 Marrakech Morocco. E-mail: soufiane@ucam.ac.ma
Received: 14 June 2001 / Accepted: 15 December 2001 / Published: 20 December 2001

The alkoxycarbonylation of terpenic olefins which affords the corresponding esters is an elegant synthesis [1-2]. Morover, the metal-complex-catalyzed hydrocarboxylation is among the most extensively investigated processes in homogeneous catalysis[3].
In a flask fitted with a reflux condenser was placed PdCl2 (34 mg, 0.19 mmol), Cu(OAc)2 (1.1 g, 5.5 mmol) and p-Toluenesulfonic acid (PTSA) (69.6 mg, 0.36 mmol), and the atmosphere was replaced with CO/O2 (1:1). 15 ml of methanol were added and the mixture was stirred for 15 min at room temperature under atmospheric pressure of CO/O2. 3-Isopropenyl-6-methyl-cyclohexene, 1, (3.6 mmol ) prepared by pyrolysis of 2-carene at high temperature (>220 °C) was added. The resulting mixture was further stirred at room temperature and at CO/O2 was bubbled in for 5 days. The reaction was monitored by GC (conversion = 94%).
At the end of the reaction, the solvent was removed by rotary evaporation. The residue was purified by using silica gel chromatography column with Hexane/AcOEt (96:4) to afford 2 as colorless oil (46%).
1H-NMR (300 MHz, CDCl3): 5.72 (1H, dd, J1=3.26 Hz/J2=1.31Hz); 3.6 (3H, S, O-CH3); 1.07 (3H, d, J=7.18 Hz, CH3); 1.01(3H, d, J=6.53 Hz, CH3).
13C-NMR (100 MHz, CDCl3): 202.2 (C=O); 172.1 (C=O); 167.4 (=C); 124 (=CH); 51.6 (OCH3); 40.9 (-CH); 39.3 (-CH2); 37.5 (-CH); 30.7 (-CH2); 27.1 (CH2); 19 (CH3); 14.9 (CH3).
M.S: m/z 210 (M+); 151 (59); 109 (42).
References
1. Naigre, R.; Chenal, T.; Ciprès, I.; Kalck, P.; Daran,
J.C.; Vaissermann, J. J. Org. Chem. 1994, 480, 91-102.
2. Chenal, T.; Ciprès, I.; Jenck, J.; Kalck, P.; Peres,
Y.
J. Mol .Catal. 1993, 78, 351-366.
3. Tkatchenko, I. Comprehensive Organometallic Chemistry,
Vol. 8, Pergamon, Oxford, (1982), 101-223.
Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI, Basel, Switzerland. All rights reserved. Molecules website http://www.mdpi.org/molecules/