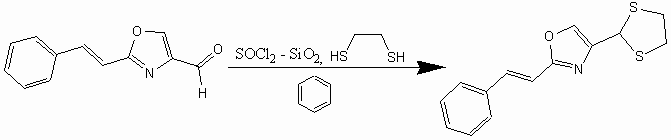
2-(2'-Cinnamyl-1',3'-oxazolyl)-1,3-dithiolane
Fahim Ahmed
Department of Chemistry, Marquette University, P O Box 1881, Milwaukee,
WI 53201-1881, USA
E-mail: [email protected]
Received: 27 October 2001 / Accepted: 15 December 2001 / Published: 20 December 2001
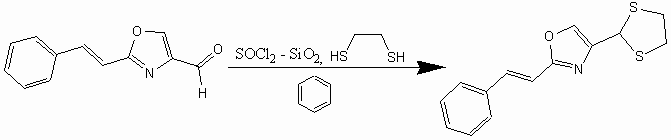
To a mixture of 2-cinnamyl-1,3-oxazole-4-carboxaldehyde [1,2] (2.8 g, 14 mmol) and ethanedithiol (2.3 g, 21 mmol) in methylene chloride (100 ml) was added boron trifluoride etherate (2.8 g, 21 mmol). The reaction mixture was stirred overnight at room temperature. The reaction mixture was washed with aqueous sodium hydroxide (5%, 50 ml), and aqueous phase was extracted with ether. The combined organic layers were dried (MgSO4), and concentrated. The residue was purified by column chromatography (SiO2, 3:1 hexanes/ethyl acetate) to give 2-(2'-cinnamyl-1',3'-oxazolyl)-1,3-dithiolane as a light yellow solid (2.25 g, 66%).
M.p. 78-82 °C.
TLC (Hexane/EtOAc 5:1): Rf 0.21
1H NMR (CDCl3): 7.55 (d, J=16.3, 1H); 7.49-7.44 (m, 3H); 7.39-6.31 (m, 3H); 6.89 (d, J=16.3, 1H); 5.52 (s, 1H), 3.34 (m, 4H).
13C NMR (CDCl3): 163.0, 144.7, 137.3, 136.0, 135.3, 129.9, 129.5, 127.8, 114.4, 47.5, 39.6.
IR (neat): nmax 3149, 2934, 2919, 1716, 962 cm-1.
Anal.calc. for C14H13NOS2.H2O (293.39): C 57.31, H 5.15, N 4.77; found: C 57.15, H 4.77, N 4.36.
Acknowledgment: The Financial support of the National Institute of Health (GM-42641) and the Marquette University Graduate School is acknowledged. The author would like to thank Dr. William A. Donaldson (E-mail: [email protected]) for his encouragement.
Reference
1. Evans, D.A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
2. Ahmed, F. 2-Cinnamyl-1,3-oxazole-4-carboxaldehyde p-Toluenesulfonyl Hydrazone. Molecules2001, 6, M269.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/