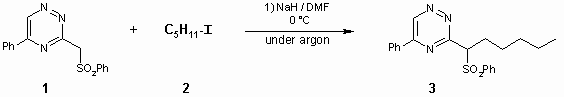
5-Phenyl-3-[1-(phenylsulphonyl)-hexyl]-1,2,4-triazine
Danuta Branowska, Stanislaw Ostrowski and Andrzej Rykowski*
Institute of Chemistry, University of Podlasie, ul. 3 Maja 54, PL-08-110
Siedlce, Poland
E-mail: [email protected]
Received: 20 November 2001 / Accepted: 15 December 2001 / Published:
20 December 2001
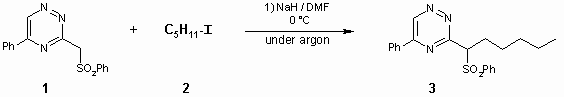
As part of ongoing research programme we synthesised the title compound as valuable intermediate for intermolecular cycloaddition reactions, leading to biologically active heterocycles.
In a round-bottom flask (25 mL), the suspension of NaH (27 mg, 1.1 mmol; washed with Et2O from 60% oil suspension) in DMF (3 ml), cooled to 0°C, was stirred for 15 min under argon. Then, the 5-phenyl-3-phenylsulphonylmethyl-1,2,4-triazine (1) [1] (312 mg, 1.0 mmol) and 1-iodopentane (200 mg, 1.0 mmol) in DMF (3 ml) was added via syringe at 0°C during a period ca 5 min. The reaction mixture was stirred at 0°C for ca 2 - 4 h until completion (TLC monitoring; CHCl3). The mixture was poured onto water with ice, the precipitate was filtered and the crude product was purified by column chromatography using CHCl3/n-hexane mixture (100:1) as eluent. Yield of pure 5-phenyl-3-[1-(phenylsulphonyl)-hexyl]-1,2,4-triazine (3) – 343 mg (90%). An analytical sample was recrystallized from ethanol/water mixture to give yellow solid.
M.p. 165-166°C (EtOH-H2O).
IR (KBr, cm-1): 2965 & 2880 (CH2), 1315 & 1160 (SO2).
1H NMR (CDCl3, 200 MHz): 0.75-0.93, 1.10-1.40, and 2.30-2.62 (3 x m, 11 H, C5H11), 4.85 (dd, J1 = 10.8 Hz, J2 = 4.4 Hz, 1 H, CH(SO2Ph)), 7.40-7.74 (m, 7 H, H-Ar), 8.07 (apparent d, J = 7.9 Hz, 2 H, H-Ar), 9.58 (s, 1 H, H-triazine).
MS (EI), m/z (% rel. int.): 381 (<1, M+.), 317 (20), 261 (6), 260 (27), 241 (6), 240 (34), 184 (7), 102 (100), 77 (20), 51 (6).
LSIMS(+), m/z (% rel. int.): 382 (100, M+H), 240 (14).
HR-LSIMS Calcd for C21H24N3SO2 (M+H): 382.1589; Found: 382.1579.
Reference
1. Rykowski, A.; Makosza, M. Liebigs Ann. Chem. 1988, 627.
Sample Availability: Available from the author and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/