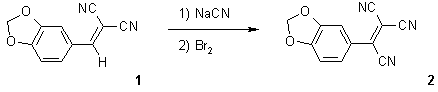
| Molbank 2002, M278 |
2-(1,3-Benzodioxol-5-yl)ethylene-1,1,2-tricarbonitrile
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia
Tel.(+966)-2-6952293,
Received:
16 January 2002 / Revised: 15 March 2002 / Accepted: 15 May 2002
Keywords: tricarbonitrile, malononitrile, piperonal
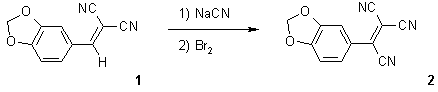
2-(1,3-benzodioxol-5-yl)ethylene-1,1,2-tricarbonitrile (2), was prepared from (1,3-benzodioxol-5-ylmethylene)malononitrile (1) and sodium cyanide, followed by oxidation using bromine [1,2]. A solution of 1 (1.0 g, 5.0 mmol) in DMF (25 mL) was cooled to 5 oC followed by dropwise addition of sodium cyanide (0.25g, 5.0 mmol) in water (10 mL). When the addition was completed, the reaction mixture was stirred at room temperature for 3 hrs. Bromine (5 mL) was added and the stirring was continued for further two hours. 1-Hexene(50 mL) was added to destroy the excess bromine. The reaction mixture was poured into water and the precipitated solid was collected by filtration. The product was recrystallizedfrom 1:1 toluene:chloroform as deep orange crystals (0.85g, 75%).
M.p.
129-131 oC (toluene:chloroform, uncorrected).
UV
λmax (nm; Chloroform)/ε (dm3.mol-1.cm-1)
344/6600, 438/11800.
IR
(cm-1;
KBr Disk) 2226 (CN), 1609 (C=C).
1H-NMR
(400 MHz; CDCl3; Me4Si, δH): 6.20 (2H, s,
OCH2O), 7.02 ( 1H, d, J = 9.72Hz), 7.59 (1H, s, H-2), 7.68 (1H, d, J
= 8.2Hz, H-6).
13C-NMR
(δC): 88.24 (OCO), 103.29, 107.92, 109.64, 111.72, 111.77, 113.78, 122.97, 128.59, 140.06, 149.33, 154.73.
Elemental
Analysis: Calculated for C12H5N3O2
(223.15): C 64.58%, H 2.24%, N 18.83%; found : C 64.36%, H 2.42%, N 18.98%.
References
1.
Lapworth, A.; McRae, J. A. J. Chem. Soc. 1922, 121,
2825.
2. Lapworth, A.; Baker, W. J. Chem. Soc. 1925, 127, 560.
© 2002 MDPI. All rights reserved.