| Molbank 2002, M279 |
2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine
Carbothioamide
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia
Tel.(+966)-2-6952293
Received:
16 January 2002 / Revised: 15 March 2002 / Accepted: 15 May 2002
Keywords: phenylhydrazine carbothioamide, thymol, 2-isopropyl-5-methylphenoxyacyl hydrazide
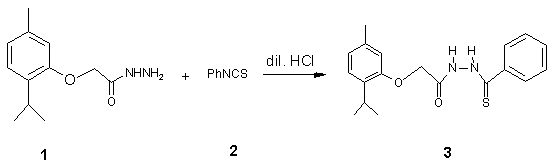
The phenylthiosemicarbazide derivative 3 was prepared from 2-isopropyl-5-methylphenoxyacyl hydrazide (1) and phenyl isothiocyanate (2) [1,2]. A mixture of 1 (0.5 g, 2.25 mmol) and 2 (0.30 g, 2.25 mmol) in aqueous hydrochloric acid (15 mL, 50%) was heated under reflux for 3 hours. The reaction mixture was cooled to room temperature and the precipitate thus formed was filtered, washed with copious amount of water and recristallized from ethanol to give 3 as white cristals. (0.71 g, 88%).
M.p.
156-158 oC
(EtOH, uncorrected).
UV lmax
(nm; Acetone)/e
(dm3.mol-1.cm-1) 274/17090, 355/4200.
IR
nmax (cm-1;
KBr Disk) 3292, 3234 (NH), 2657 (SH), 1674 (C=O), 1553 (NH bending).
1H-NMR
(400 MHz; CDCl3; Me4Si) dH
0.95 (6H, d, 2CH3), 2.03 (3H, s CH3), 3.17 (1H, m, CH),
4.36 (2H, S, CH2O), 6.40 (1H, s), 6.51 1H, d, J = 7.63 Hz),
6.83 (1H, d, J =7.73 Hz), 6.88 (1H, d, J =7.35 Hz), 7.05 (2H, t, J
=7.85 Hz), 7.24 (2H, d, J =7.5 Hz).
13C-NMR
(dC)
21.44,
23.24, 26.50, 67.15 (CH2O), 112.5, 122.63, 124.0, 125.47, 126.3,
128.8,134.38, 136.7, 138.8154.58.
Elemental
Analysis: Calculated for C19H23N3O2S
( 357.22): C 63.88, H 6.44, N 11.77; found : C 63.67, H 6.56 , N 11.62.
References
1.
Trivedi, S.; Kubavate, H.; Parekh, H. Indian J. Chem. 1994, 33,
295.
2.Vashi,
B.
S.; Mehta, S., Shah, V. H. Indian J. Chem. 1996, 35,
11.
© 2002 MDPI. All rights reserved.