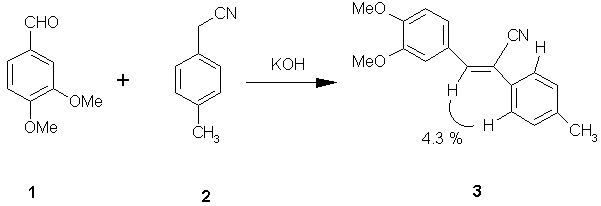
| Molbank 2002, M281 |
(2Z)-3-(3,4-Dimethoxyphenyl)-2-(4-methylphenyl)acrylonitrile
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia
Tel.(+966)-2-6952293,
Received:
16 January 2002 / Revised: 15 March 2002 / Accepted: 15 May 2002
Keywords:
acrylonitrile, 3,4-dimethoxybenzaldehyde,
4-methylphenylacetonitrile, Knoevenagel condensation

UV lmax
(nm; EtOH)/e
(dm3.mol-1.cm-1) 335/25000, 249/24500.
IR
(cm-1; KBr Disk) 2207 (CN), 1615 (C=C).
1H-NMR
(400 MHz; CDCl3, Me4Si, dH):
7.70 (1H, d, J= 2 Hz, H-2 ), 7.54 (2H, d, J= 8.2 Hz, H-2 ), 7.41 (1H, s,
olefinic proton), 7.34 (1H, dd, J= 8.4, J 2 Hz, H-6), 7.24 (2H, d, J=8.03 Hz,),
6.91 (1H, d, J= 8.4 Hz), 3.96, 3.93 (6H, s, 2xMeO), 2.38 (3H, s, MePh).
13C-NMR
(dC):
21.2, 55.95, 108.6, 110.5, 110.8, 118.8, 124.2, 125.6, 126.8, 129.7, 131.9,
138.9, 141.1, 148.9 and 150.9.
Elemental
Analysis: Calculated for C18H17O2N
(279.207): C 77.43%, H 6.09%, N 5.02%; Found: C 77.25%, H 4.96%, N 5.21%.
References
1.
Jones, G. Org. React. 1967, 15, 204.
2.
Stewart, J. T.; Kim, M. J. Chem. Eng. Data 1987, 32, 387.
© 2002 MDPI. All rights reserved.