| Molbank 2002, M286 |
2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline
Zhong-Ming
Wang
a
, Yong-Mei Hao
b, Jian-Zhong
Liu
a
and Lian-Nian Ji
a*
a The Key Laboratory of Gene Engineering of Ministry of Education /
Department of Chemistry, Zhongshan University, Guangzhou 510275, China. Tel. +86-20-84036461
b College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
Received: 25 April 2002 / Accepted: 29 May 2002 / Published: 20 February 2003
Keywords: polypyridine
ligands, phenanthroline, benzil

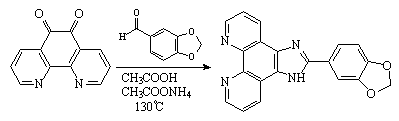
1,10-Phenanthroline-5,6-dione
was prepared by a previously published procedure [1]. The title compound was
synthesized according to the method for the preparation of imidazole rings
established by Steck and Day [2]. A mixture of 3,4-(methylenedioxy)benzaldehyde
(8 mmol), 1,10-phenanthroline-5,6-dione (5 mmol), ammonium acetate (100 mmol)
and glacial acetic acid (20 cm3) was refluxed with stirring at 130°C
for about 1.5 hour, then cooled to room temperature and diluted with water (ca.
20 cm3). Dropwise addition of concentrated aqueous ammonia with
stirring gave a yellow precipitate, which was filtered and washed with water and
acetone. The crude product was purified by silica gel filtration (60-100 mesh,
ethanol as eluent). The principal yellow band was collected. After most of the
ethanol solvent was removed under reduced pressure, the amorphous yellow solid
was filtered and washed with acetone and diethyl ester, then dried in vacuum.
Yield 1.03 g, 58%.
Elemental
analyses: Found C, 69.23; H, 3.64; N, 15.87. Calc. For C20H12N4O2×0.5H2O,
C, 68.76; H, 3.75; N, 16.04.
1H
NMR (500 MHz, (CD3)2SO): d
13.50 (br, s, 1H), 9.02 (dd, 2H), 8.90 (d, 2H), 7.85 (m, 2H), 7.80 (m, 2H), 7.12
(d, 1H), 6.19 (s, 1H).
IR
(KBr, cm-1): 463 (w), 547 (w), 621 (m), 740 (s), 813 (s), 879 (m),
937 (m), 963 (m), 1036 (s), 1073 (m), 1116 (m), 1190 (w), 1225 (s), 1258 (s),
1350 (s), 1397 (m), 1437 (m), 1471 (s), 1500 (m), 1562 (m), 1604 (m), 2898 (w),
2963 (w), 3001 (w), 3066 (w), 3105 (w), 2688-3105 (br), 3423 (br, m).
FAB-MS
([M+1]+): 341, 50%.
Acknowledgement: Support for this research provided by the National Natural Science Foundation of China and the National Science Foundation of Guangdong Province.
References
1.
Paw, W.; Eisenberg, R. Inorg. Chem. 1997,
36, 2287.
© 2002 MDPI. All rights reserved.