| Molbank 2002, M288 |
2-Bromo-N1, N1, N4, N4-tetramethyl-benzene-1,4-diamine
Harald Zieg, Wolfgang Pitsch and Burkhard
Koenig*
Institut fuer Organische Chemie, Universitaet Regensburg, D-93040 Regensburg, Germany
Fax (+49) 941 943 171; E-mail: [email protected]
Received: 4 March 2002 / Accepted: 15 March 2002 / Published: 24 February 2003
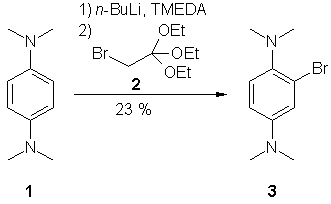
Compound 3 has been reported in a
patent1 as a catalyst for hydrogen peroxide bleaching in photographic processing methods, but no synthesis or spectroscopic data are available in the chemical literature. We report here the synthesis and characterization of the compound.
A mixture of N,N,N',N'-tetramethyl-benzene-1,4-diamine (3.1 g, 18.9 mmol) (1), n-BuLi (22.7 mmol, 16.2 ml; 1.4 mol/L in hexane) and TMEDA (2.8 ml, 18.9 mmol) was heated to reflux for 45 min. A white precipitate is observed. The reaction mixture was cooled down to 0 °C, the solvent was decanted from the residue and 50 ml of
THF were added. After addition of 2-bromo-1,1,1-triethoxy-ethane (5.0 g, 21 mmol)
(2) the reaction mixture was heated to reflux for 15 min and stirred at room temp. overnight. The reaction was quenched with aqueous sat.
NH4Cl solution (100 ml), diluted with ethyl acetate, the organic phase was washed with water, dried over
Na2SO4 and the solvent was removed in vacuo. The crude product was purified by column chromatography on silica [petrol
ether (60/70) / ethyl acetate 3:1; Rf = 0.56] to yield 1.03 g (23 %) of 3; brown solid, mp. 35 °C.
IR (KBr): 2979 cm-1, 2937, 2890, 2853, 2821, 2788, 1605, 1507, 942, 811, 678.
UV/Vis (CH3CN): lmax (log e) = 210 nm (4.333), 266 (4.147), 322 (3.445).
1H NMR (400 MHz, CDCl3): d = 2.70 (s, 6H), 2.88 (s, 6H), 6.65 (dd, 3J = 8.8 Hz, 4J = 2.9 Hz, 1H), 6.94 (d, 4J = 2.9 Hz, 1H), 7.02 (d, 3J = 8.8 Hz, 1H).
13C NMR (100 MHz, CDCl3): d = 40.81 (+), 44.92 (+), 112.49 (+), 117.65 (+), 120.90 (Cquat), 121.06 (+), 141.71 (Cquat), 147.91 (Cquat).
MS (70 eV), m/z (%): 242 (100) [M+], 227 (76) [M+-CH3].
References and Notes
1. O'Toole, T. R., US Patent 1997, Cont.-in-part of U.S. Ser. No. 362,384.
Sample availability: available form the authors.
© 2002 MDPI. All rights reserved.