| Molbank 2003, M300 |
Methyl 8-Acetyl-labdanolate
(-)-(3S)-5-((1R,2R,4aS,8aS)-2-Acetoxy-2,5,5,8a-tetramethyldecahydro-1-naphthalenyl)-3-methylpentanoic Acid Methyl Ester
Juan M. Castro, Sofia Salido, Joaquin Altarejos*, Manuel Nogueras and Adolfo Sanchez
Departamento de Química Inorgánica y Orgánica, Facultad de Ciencias Experimentales, Universidad de Jaén, 23071 Jaén, Spain
Tel.: 34-953-002743, fax: 34-953-012141, E-mail: [email protected]
Received: 12 December 2001 / Accepted: 19 September 2002 / Published: 8 March 2003
Keywords: Diterpenes, labdanes, methyl labdanolate, acetylation, tertiary alcohol
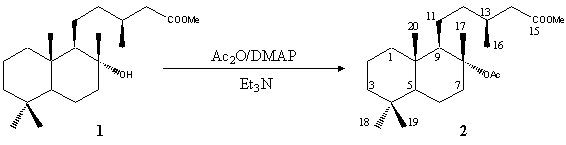
Acetic anhydride (0.53 mL, 5.61 mmol) and N,N-dimethylaminopyridine (17 mg, 0.14 mmol) were added to a stirred solution of the alcohol
1 (442 mg, 1.31 mmol) in freshly distilled triethylamine (1.3 mL) [1]. The reaction was mantained between 35-40 ºC for 72 h and, then, water was added (25 mL) and the mixture extracted with
Et2O (3×40 mL). The combined organic layers were washed with 2N HCl (25 mL), saturated
Na2CO3 (25 mL) and brine (3×25 mL). The organic phase was dried over anhydrous
Na2SO4 and the solvent evaporated under reduced pressure to yield a residue (539 mg) which was purified by flash chromatography on silica gel, using a 3:2
hexane/Et2O mixture as eluent, to give the pure title compound 2 (296 mg, 0.78 mmol, 60%).
Mp: 81.0-82.3 ºC (white crystals, from hexane).
[a]D = -31.8º (c 0.97 cg·mL-1, CHCl3).
IR (neat, n, cm-1): 1735 (COOMe), 1723 (OAc), 1252, 1217, 1148 (COOMe, OAc).
1H NMR (300 MHz, CDCl3, d, ppm): 0.78 (3H, s,
Meb-4), 0.82 (3H, s, Me-10), 0.86 (3H, s,
Mea-4), 0.95 (3H, d, J=6.6 Hz, Me-13), 1.44 (3H, s, Me-8), 1.93 (3H, s, OAc), 0.98-2.00 (16H, m, H-1,2,3,5,6,7a,9,11,12,13), 2.12 (1H, dd, J=14.7 Hz, 7.8 Hz, H-14), 2.31 (1H, dd, J=14.7 Hz, 6.5 Hz, H'-14), 2.62 (1H, dt, J=12.5 Hz, 3.3 Hz, Hb-7), 3.66 (3H, s, OMe).
13C NMR (75 MHz, CDCl3, d, ppm): 39.48 (C-1), 18.27 (C-2), 41.85 (C-3), 33.03 (C-4), 55.55 (C-5), 19.93 (C-6), 38.66 (C-7), 87.88 (C-8), 58.90 (C-9), 39.31 (C-10), 22.99 (C-11), 39.84 (C-12), 31.02 (C-13), 41.56 (C-14), 173.66 (C-15), 19.82 (C-16), 20.36 (C-17), 33.26 (C-18), 21.36 (C-19), 15.64 (C-20), 51.26 (OMe), 170.14 (OAc), 22.82 (OAc).
Acknowledgements: We wish to thank the Junta de Andalucía for financial support and the Ministerio de Educación, Cultura y Deporte for a Fellowship to J. M. Castro.
References and Notes
1. Urones, J. G.; Basabe, P.; Marcos, I. S.; González, J. L.; Jiménez, V.; Sexmero, M. J.; Lithgow, A. M. Ambergris Compounds from Labdanolic Acid.
Tetrahedron 1992, 48, 9991-9998.
Sample availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.