| Molbank 2003, M305 |
4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one
Hussein F. Zohdi a* and Nora M. Rateb b
a Department of Chemistry, Faculty of Science, United Arab Emirates University, P.O.Box 17551 Al-Ain, UAE
b Department of Chemistry, Faculty of Science, Cairo University, Giza, Egypt
Received: 16 June 2002 / Accepted: 30 September 2002 / Published: 11 March 2003
Keywords: Trifluoromethyl, Dihydropyrazol-3-one, Tolyldiazonium chloride
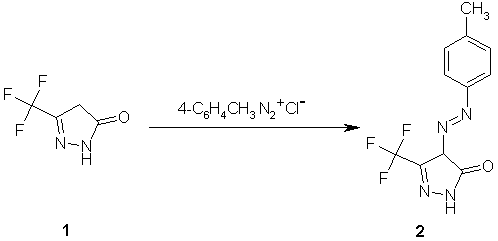
To a cold solution of 5-trifluoromethyl-2,4-dihydropyrazol-3-one [1,2] (0.76 g, 5 mmol) in ethanol (25 ml) containing sodium acetate (0.82
g, 10 mol), p-tolyldiazonium chloride [ca. 5 mmol; prepared by adding a solution of sodium nitrite
(5 mmol / 2 ml H2O) to an ice cold solution of p-toluidine in 2 ml conc. HCl] was added dropwise with stirring at
0-5°C. The reaction mixture was stirred at room temperature for three hours and the precipitated crude product was filtered, washed with water, dried and crystallized from ethanol to give
1.0 g (74%) of 2 as orange crystals.
M.p. 180-181°C
IR (KBr, cm-1): 3210 (NH), 1670 (CO pyrazolone).
MS (m/z): 270.
1H-NMR (250 MHz, DMSO-d6): 2.31 (s, 3H, CH3);
2.45 (s, 1H, CH); 7.21 (dd, 4H, aromatic CH); 12.58 (s, 1H, NH).
13C-NMR (75 MHz, CDCl3): 24.2 (CH3); 51.5 (CH);
122.2 (2 aromatic carbons); 129.7 (2 aromatic carbons); 134.3, 143.7 (quaternary aromatic carbons);
135.7 (q, CF3); 153.6 (C=N); 173.1 (CO).
References and Notes
1. Zohdi, H. F.; Elghandour, A. H. H.; Rateb, N. M.; Sallam, M. M. M. J. Chem. Res. 1992, (S) 396, (M) 3015.
2. Zohdi, H. F.; Rateb, N. M. Molecules 2001,
6, M261.
Sample Availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.