| Molbank 2003, M307 |
2-(b-D-Ribofuranosyl)-4-(p-tolylazo)-5-trifluoromethyl-2,4-dihydropyrazol-3-one
Abdelfattah Haikal*, Hussein F. Zohdi and Zahra Badi
Department of Chemistry, Faculty of Science, United Arab Emirates University, P.O.Box 17551 Al-Ain, UAE
E-mail: [email protected] and [email protected]
Received: 16 June 2002 / Accepted: 30 September 2002 / Published: 11 March 2003
Keywords: Ribofuranose, Nucleosides, Dihydropyrazol-3-one
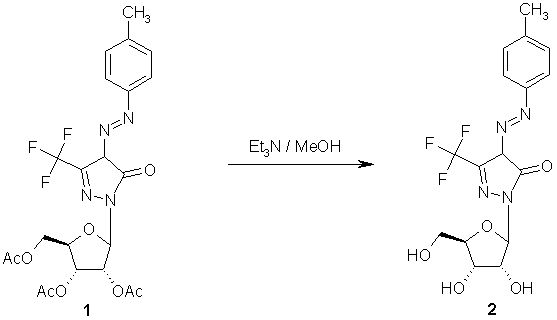
The desired compound 2 was obtained by complete deprotection of the acetylated nucleoside
1 [1] using triethylamine [2]. To a solution of 1 (0.8g, 1.5 mmol) in methanol (25 ml) was added triethylamine (2 ml). The mixture was stirred at room temperature and the reaction was followed by tlc. After complete deprotection (24 hours), the reaction mixture was evaporated and coevaporated with methanol (3 x 30 ml), then chromatographed over silica gel using
CH2Cl2/MeOH (95:5 v/v) to give 0.55 g (90%) of 2 as yellow powder.
Rf: 0.30 (CH2Cl2/MeOH, 95/5 v/v).
UV (lmax , 95% ethanol): 384nm
IR (KBr, cm-1): 3414 (OH), 1662 (CO pyrazolone).
MS (m/z): 402.
1H-NMR (250 MHz, DMSO-d6): 2.38(s, 3H, CH3); 2.40(s, 1H, CH); 3.73(dd, 1H, H-5` J5`,4`=2.4 Hz) 3.91-3.96(dd, 1H, H-5`` J5``,4`=2.4 Hz); 4.23-4.24(m, 1H, H-4`); 4.53(t, 1H, H-3` J3`,2`=3.66 Hz); 4.75(t, 1H, H-2` J2`,3`=5.13 Hz); 5.94(d, 1H, H-1`, J1`,2`=4.92 Hz); 7.21-7.37(m, 4H, aromatic CH).
13C-NMR (75 MHz, DMSO-d6): 22.0(CH3); 48.9(CH); 63.08(C-5`), 71.71(C-3`); 73.90(C-2`); 85.63(C-4`); 88.08(C-1`); 116.9 (2 aromatic carbons), 121.0, 122.0 (2 aromatic carbons), 130.4, 144.0 (2 quaternary aromatic carbons); 137.7(q,
CF3); 148.5(C=N); 173.5(CO).
References and Notes
1. Haikal, A.; Zohdi, H. F.; Badi, Z. Molbank 2003, M0306.
2. Zohdi, H. F.; Haikal, A. Molecules 2001, 6, M263.
Sample Availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.