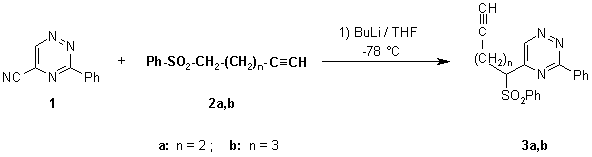
| Molbank 2003, M314 |
3-Phenyl-5-[1-(phenylsulphonyl)-pent-4-yn]-1,2,4-triazine
and 3-Phenyl-5-[1-(phenylsulphonyl)-hex-5-yn]-1,2,4-triazine
Danuta Branowska, Stanislaw Ostrowski and Andrzej Rykowski*
Institute of Chemistry, University of Podlasie, ul. 3 Maja 54, PL-08-110
Siedlce, Poland
E-mail: [email protected]
Received: 26 September 2002 / Accepted: 15 November 2002 / Published: 24 March 2003
Keywords: 1,2,4-triazines, carbanions, sulphones
As part of ongoing research programme we synthesised the title compounds as valuable intermediates for intramolecular cycloaddition reactions, leading to biologically active heterocycles.
In a three-necked round-bottom flask (50 mL), equipped with a thermometer, a solution of 4-pentyn phenyl sulphone (2a) or 5-hexyn phenyl sulphone (2b) (1.0 mmole) [1] in dry THF (6 mL) is cooled to -78°C. To the stirred solution, BuLi (1.5 mmole, 0.94 mL of 1.6 N solution in hexane) was added gradually via syringe in such a rate to keep the temperature below -35°C. The mixture was cooled again to -78°C and a solution of 3-phenyl-1,2,4-triazine-5-carbonitrile [2] (1, 182 mg, 1.0 mmole) in dry THF (10 mL) was added dropwise via syringe during a period ca 10 min. It was stirred for ca 2 h and then allowed to warm to room temperature. To this mixture saturated aqueous NH4Cl solution (20 mL) was added and it was extracted with CH2Cl2 (2 x 20 mL), dried over MgSO4, and evaporated to dryness. Column chromatography purification (silica gel 200-300 mesh, eluent: CHCl3) yielded 190 mg (52.5%) of pure 3-phenyl-5-[1-(phenylsulphonyl)-pent-4-yn]-1,2,4-triazine (3a) as a yellow solid (or 140 mg of 3-phenyl-5-[1-(phenylsulphonyl)-hex-5-yn]-1,2,4-triazine (3b), 37%).
3-Phenyl-5-[1-(phenylsulphonyl)-pent-4-yn]-1,2,4-triazine (3a):
M.p. 86-87ºC.
IR (KBr, cm-1): 3280 (C![]() C-H), 1325 & 1165
(SO2).
C-H), 1325 & 1165
(SO2).
1H NMR (CDCl3, 200 MHz): 1.97 (t, J = 2.6 Hz, 1 H, C![]() CH), 2.00-2.20 (m, 1 H), 2.34-2.52 (m, 1 H), and 2.55-2.68 (m, 2 H) [2 x
CH2], 4.65 (dd, J1 = 9.0 Hz, J2 = 5.5 Hz, 1 H, CH(SO2Ph)), 7.41-7.67 (m, 8 H, H-Ar), 8.21-8.27 (m, 2 H, H-Ar), 9.23 (s, 1 H,
H-triazine).
CH), 2.00-2.20 (m, 1 H), 2.34-2.52 (m, 1 H), and 2.55-2.68 (m, 2 H) [2 x
CH2], 4.65 (dd, J1 = 9.0 Hz, J2 = 5.5 Hz, 1 H, CH(SO2Ph)), 7.41-7.67 (m, 8 H, H-Ar), 8.21-8.27 (m, 2 H, H-Ar), 9.23 (s, 1 H,
H-triazine).
MS (EI), m/z (% rel. int.): 363 (2, M+.), 362 (2), 311 (28), 222 (28), 194 (48), 184 (23), 169 (11), 167 (13), 141 (12), 125 (11), 104 (35), 91 (100), 77 (45), 65 (35), 51 (13).
HR-MS (EI) Calcd for C20H17N3SO2: 363.1042;
Found: 363.1044.
3-Phenyl-5-[1-(phenylsulphonyl)-hex-5-yn]-1,2,4-triazine (3b):
M.p. 102-103°C.
IR (KBr, cm-1): 3295 (C![]() C-H), 1370 & 1170
(SO2).
C-H), 1370 & 1170
(SO2).
1H NMR (CDCl3, 200 MHz) 1.40-1.62 (m, 2 H, CH2), 1.95 (t, J = 2.6 Hz, 1 H,
C![]() CH), 2.23 (td, J1 = 6.8 Hz,
J2 = 2.6 Hz, 2 H,
CH2), 2.47-2.61 (m, 2 H, CH2), 4.43 (dd, J1 = 9.9 Hz,
J2 = 5.3 Hz, 1 H,
CH(SO2Ph)), 7.40-7.66 (m, 8 H, H-Ar), 8.20-8.28 (m, 2 H, H-Ar), 9.26 (s, 1 H,
H-triazine).
CH), 2.23 (td, J1 = 6.8 Hz,
J2 = 2.6 Hz, 2 H,
CH2), 2.47-2.61 (m, 2 H, CH2), 4.43 (dd, J1 = 9.9 Hz,
J2 = 5.3 Hz, 1 H,
CH(SO2Ph)), 7.40-7.66 (m, 8 H, H-Ar), 8.20-8.28 (m, 2 H, H-Ar), 9.26 (s, 1 H,
H-triazine).
MS (EI), m/z (% rel. int.): 377 (2, M+.), 376 (4), 311 (11), 208 (40), 105 (100), 104 (34), 103 (29), 79 (33), 77 (48), 65 (11), 51 (9).
HR-MS (EI) Calcd for C21H19N3SO2: 377.1198;
Found: 377.1192.
References
1. Prepared from methyl phenyl sulphone and corresponding 4-iodo-1-butyne or 5-iodo-1-pentyne,
under argon in dry THF at -78°C to r.t., in presence of BuLi (modified procedure of: Taylor,
E.C.; Macor, J.E.; French, L.G. J. Org. Chem. 1991, 56, 1807).
2. a) Huang, J.J. J. Heterocycl. Chem. 1985, 22, 1329. b) Rykowski, A.; Branowska, D.; Makosza, M.; van Ly, P. J. Heterocycl. Chem. 1996, 33, 1567.
© 2003 MDPI. All rights reserved.