| Molbank 2003, M315 |
(±)-1-(4-Hydroxy-3-methoxyphenyl)-3-butanol
Guy L. Plourde
University of Northern British Columbia, Department of Chemistry, 3333 University Way, Prince George, British Columbia, Canada, V2N 4Z9
Tel: 250-960-6694, Fax: 250-960-5545, E-mail: [email protected]
Received: 7 July 2002 / Accepted: 15 October 2002 / Published: 24 March 2003
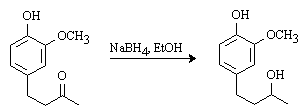
The discussion and purpose for the synthesis of this compound has been reported elsewhere [1]. To a cold (0°C) solution of 1-(4-hydroxy-3-methoxyphenyl)-3-butanone (735 mg, 3.8 mmol) in EtOH (35 mL) was added sodium borohydride (145 mg, 3.8 mmol, 1 eq). The solution was stirred at 0°C for 30 min., then at room temperature for 2 h. 10% HCl (15 mL) was added and the solution was stirred at room temperature for 2 h. The solution was concentrated in vacuo and the aqueous residue was extracted with ethyl acetate (3 x 20 mL). The organic fractions were combined, dried (MgSO4) and the solvent was evaporated in vacuo. Chromatography on silica gel (40% EtOAc/hexanes) afforded a colorless oil (600 mg, 81%).
IR (neat)
cm-1: 3311 (OH)
1H-NMR (CDCl3) d: 1.24 (d, 3H, J=6.2 Hz,
H-4), 1.76 (m, 2H, H-2), 2.68 (m, 2H, H-1), 3.82 (m, 1H, H-3), 3.89 (s, 3H, OCH3), 5.53 (s, 1H, exchangeable with
D2O, OH), 6.67 (d, 1H, J=7.4 Hz, ArH-6), 6.72 (s, 1H, ArH-2), 6.84 (d, 1H, J=7.4 Hz,
ArH-5).
13C-NMR (CDCl3) d: 23.7 (C-4), 32.1
(C-2), 41.3 (C-1), 56.1 (OCH3), 67.8 (C-3), 111.1 (ArC-2), 114.4 (ArC-5), 121.1
(ArC-6), 134.2 (ArC-1), 143.9 (ArC-4), 146.6 (ArC-3).
MS m/e (rel %): 196 [M+] (97), 178 (12), 163 (27), 138 (86), 137 (100), 134 (42), 123 (22), 106 (16), 91 (15).
Anal. calc. for C11H16O3: C 67.31, H 8.74; found: C 67.03, H 8.55.
Acknowlegment
The author is thankful for the financial support provided by the University of Northern British Columbia.
Reference
1. Plourde, G.L. Tetrahedron Letters 2002, 43, 3597-3599.
© 2003 MDPI. All rights reserved.